- Title
-
Insights into the role of Fsh signaling in ovarian differentiation of chorionic gonadotropin α ( cgα)-deficient zebrafish
- Authors
- Shi, C., Zhang, Y., Lu, Y., Lou, Q., Shang, G., Peng, X., Dai, X., Jin, X., He, J., Zhai, G., Yin, Z.
- Source
- Full text @ Zool Res
|
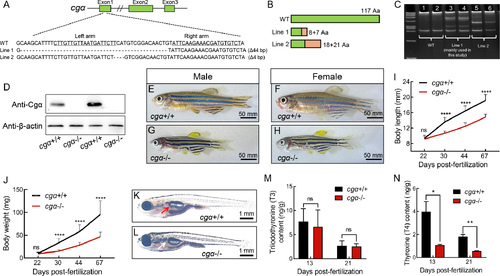
Generation and characterization of cgα-deficient zebrafish using TALENs A: Schematic of TALEN-mediated editing at exon 1 of the cgα locus. Underlined sequences denote the two targeting arms of TALENs. B: Diagram of WT and two truncated mutant Cgα proteins in zebrafish. Green regions represent amino acid sequences identical to WT, while orange regions indicate mismatched residues. C: Heteroduplex-based genotyping of cgα in cgα+/+ control fish (Lanes 1 and 2) and cgα+/− fish (Lanes 3 and 4 for mutant line 1; Lanes 5 and 6 for mutant line 2), amplified products from tail gDNA samples. D: Western blot analysis of Cgα in pituitary samples from control and mutant lines of cgα-deficient male zebrafish. E–H: Gross appearance of control siblings (E, control male; F, control female) and cgα-deficient fish (G, mutant male; H, mutant female) at 4 mpf. I, J: Body length (I) and weight (J) of control siblings and cgα-deficient fish from juvenile to adult stages. K, L: Gross appearance of control sibling (K) and cgα-deficient fish (L) at 16 dpf. Red arrow indicates inflated anterior swim bladder of control fish, white arrow indicates undeveloped anterior swim bladder of cgα-deficient fish. M, N: Levels of triiodothyronine (T3, M) and thyroxine (T4, N) in bodies of control and cgα-deficient fish at 13 and 21 dpf stages. ns: Not significant; *: P<0.05; **: P<0.01; ****: P<0.0001. |
|
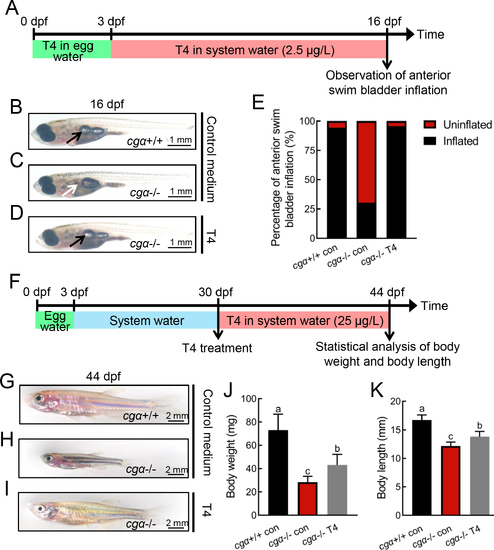
Defective swim bladder inflation and retarded growth of cgα-deficient fish rescued by exogenous thyroxine (T4) supplement A: Schematic of rearing timeline and early T4 administration treatment. Fertilized eggs were reared in egg water containing T4 from 0 to 3 dpf, and in system water containing T4 from 4 to 16 dpf. B–D: Gross appearance of fish at 16 dpf, including control (B), cgα-deficient (C), and cgα-deficient fish administered T4 (D). Black arrow indicates inflated anterior swim bladder of control (B) and cgα-deficient fish administered T4 (D), white arrow indicates undeveloped anterior swim bladder of cgα-deficient fish (C). E: Ratio of anterior swim bladder inflation in control, cgα-deficient, and cgα-deficient fish administered T4 at 16 dpf. F: Schematic of rearing timeline and late T4 administration treatment. Fish were reared in system water containing T4 from 30 to 44 dpf. G–I: Gross appearance of fish at 44 dpf, including control fingerlings (G), cgα-deficient fingerlings (H), and cgα-deficient fingerlings administered T4 (I). J, K: Body weight (J) and length (K) of control, cgα-deficient, and cgα-deficient fingerlings administered T4 at 44 dpf. dpf, days post-fertilization; T4, thyroxine. Different letters in bar chart represent significant differences. |
|
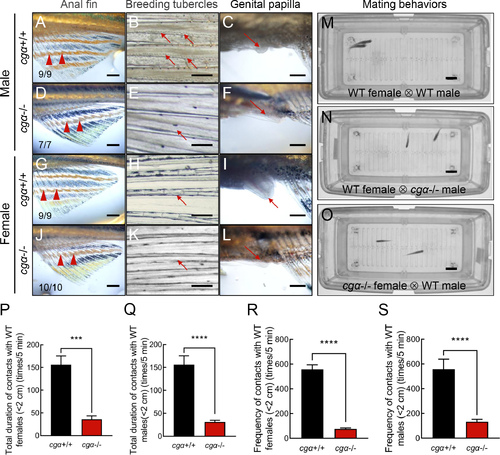
Disrupted male-typical SSCs and impaired mating behaviors in cgα-deficient zebrafish A, D, G, J: Anal fin coloration in control male (A), cgα-deficient male (D), control female (G), and cgα-deficient female (J). Black arrowheads indicate male-typical bright yellow characteristic of control males, red arrowheads indicate dark yellow characteristic of control females and both sexes of cgα-deficient fish at 4 mpf. B, E, H, K: Presence of pectoral fin BTs. Black arrows indicate pectoral fin BTs in control males (B), red arrows indicate absence of pectoral fin BTs in control females (E) and both sexes of cgα-deficient fish (H, K) at 4 mpf. C, F, I, L: Formation of genital papilla. Red arrow indicates female-typical genital papilla in control females (I), black arrows indicate absence of genital papilla in control males (C) and both sexes of cgα-deficient fish (F, L) at 4 mpf. Scale bars: 2 mm. M–O: Representative mating behavior sequences monitored using the ZebraTower system. Parallel swimming, grasping, and spawning were observed between WT males and females (M), while only transient encounters or avoidance were seen in pairing of cgα-deficient and WT fish of the opposite sex (N, O). Scale bars: 2 cm. P–S: Quantification of duration (P, tested with WT female; Q, tested with WT male) and frequency (R, tested with WT female; S, tested with WT male) of intimate contacts over 5 min (<2 cm). WT, wild-type. ***: P<0.001; ****: P<0.0001. |
|
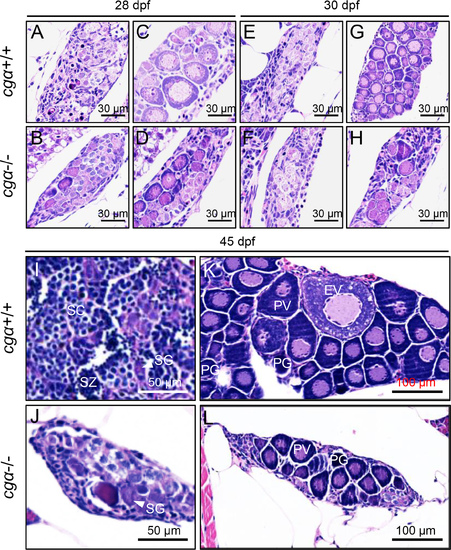
Histological analysis of gonadal development in control and cgα-deficient fish during key stages of sex differentiation A–H: No obvious gonadal differences were observed between control and cgα-deficient fish at 28 and 30 dpf. I, J: At 45 dpf, testes from cgα-deficient males were visibly smaller than those of control males, with germ cells predominantly at the spermatogonia stage. K, L: At 45 dpf, ovaries of cgα-deficient females were smaller than those of control females and contained only PV and PG oocytes. dpf, days post-fertilization; SG, spermatogonia; SC, spermatocytes; SZ, spermatozoa; PG, primary growth follicle; PV, previtellogenic follicle; EV, early vitellogenic follicle. |
|
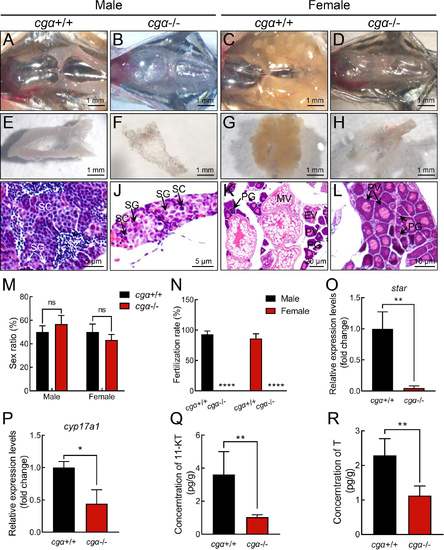
Gonadal hypoplasia in cgα-deficient zebrafish at 4 mpf A–D: Anatomical examination of gonads from control siblings (A, male; C, female) and cgα-deficient fish (B, male; D, female) at 4 mpf. E–H: Dissected gonadal tissues of control siblings (E, male; G, female) and cgα-deficient fish (F, male; H, female) at 4 mpf. I–L: Histological analysis of gonadal tissues from control siblings (I, male; K, female) and cgα-deficient (J, male; L, female) fish at 4 mpf. M: Sex ratio of control siblings and cgα-deficient fish at 4 mpf (n=20). N: Fertilization ratio of control siblings and cgα-deficient fish at 4 mpf when paired with WT fish of the opposite sex (n=8/group). O, P: Expression levels of star (O) and cyp17a1 (P) in testis tissue of control siblings and cgα-deficient fish at 4 mpf. Q, R: Levels of 11-KT (Q) and testosterone (R) from whole-body lysates of cgα-deficient male fish and control male siblings at 4 mpf. SG, spermatogonia; SC, spermatocytes; SZ, spermatozoa; PG, primary growth follicle; PV, previtellogenic follicle; EV, early vitellogenic follicle; MV, middle vitellogenic follicle; 11-KT, 11-ketotestosterone; T, testosterone. ns: Not significant; *: P<0.05; **: P<0.01; ****: P<0.0001. |
|
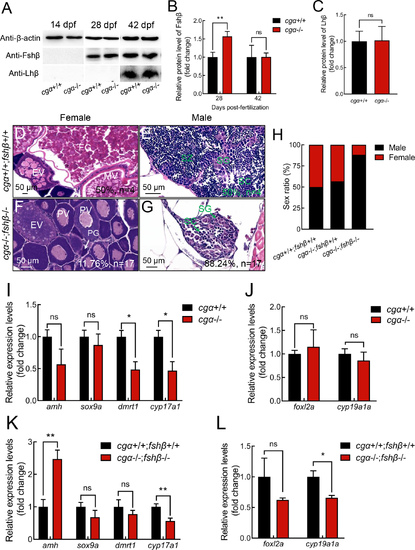
Additional fshβ depletion in cgα-deficient zebrafish skews gonadal sex differentiation towards males A: Western blot analysis of Fshβ and Lhβ proteins in head tissue containing pituitary from control and cgα-deficient fish at 14, 28, and 42 dpf. B: Fshβ protein levels were significantly increased in cgα-deficient fish at 28 dpf, but not at 42 dpf. C: Lhβ protein levels remained unchanged in cgα-deficient fish compared to controls at 42 dpf. D–G: Histological analysis of control and cgα−/−;fshβ−/− fish at 4 mpf. H: Sex ratio of control, cgα−/−, and cgα−/−;fshβ−/− fish at 4 mpf. I: Transcription of male pathway-related genes showed no significant changes in cgα-deficient male fish compared to controls at 4 mpf, including amh, sox9a, dmrt1, and cyp17a1. J: Transcription of female pathway-related genes showed no significant changes in cgα-deficient female fish compared to controls at 4 mpf, including foxl2a and cyp19a1a. K: Transcription of amh was significantly up-regulated in cgα−/−;fshβ−/− male fish compared to controls at 4 mpf, although no significant alteration in transcription of sox9a, dmrt1, and cyp17a1 was observed. L: Transcription of foxl2a and cyp19a1a showed no significant changes in cgα−/−;fshβ−/− female fish compared to controls at 4 mpf. dpf, days post-fertilization; SG, spermatogonia; SC, spermatocytes; SZ, spermatozoa; PG, primary growth follicle; PV, previtellogenic follicle; EV, early vitellogenic follicle; MV, middle vitellogenic follicle; FG, full-grown follicle. ns: Not significant; *: P<0.05; **: P<0.01. |
|
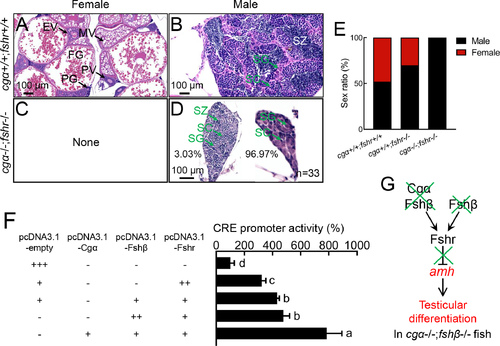
Functional analysis of Fsh signaling function in ovarian differentiation in zebrafish A–D: No ovarian differentiation was observed in cgα−/−;fshr−/− fish at 4 mpf. E: Sex ratio of cgα+/+;fshr+/+ control, fshr−/−, and cgα−/−;fshr−/− fish at 4 mpf. F: cAMP-responsive CRE-luciferase reporter assay in CHO cells transfected with zebrafish Fshr, Fshβ/Fshr, or Cgα/Fshβ/Fshr constructs demonstrated both ligand-independent (constitutive) activity of Fshr and enhanced signaling via Fshβ, with further amplification upon Cgα co-expression. G: Proposed working model of Fsh signaling in ovarian differentiation. SG, spermatogonia; SC, spermatocytes; SZ, spermatozoa; PG, primary growth follicle; PV, previtellogenic follicle; EV, early vitellogenic follicle; MV, middle vitellogenic follicle; FG, full-grown follicle. Letters in bar charts represent significant differences. Green crosses denote gene knockout or inactivation, red letters indicate up-regulation or male-biased sex ratio. CRE, cAMP response element. |