- Title
-
The intestinal microbiome and Cetobacterium somerae inhibit viral infection through TLR2-type I IFN signaling axis in zebrafish
- Authors
- Liang, H., Li, M., Chen, J., Zhou, W., Xia, D., Ding, Q., Yang, Y., Zhang, Z., Ran, C., Zhou, Z.
- Source
- Full text @ Microbiome
|
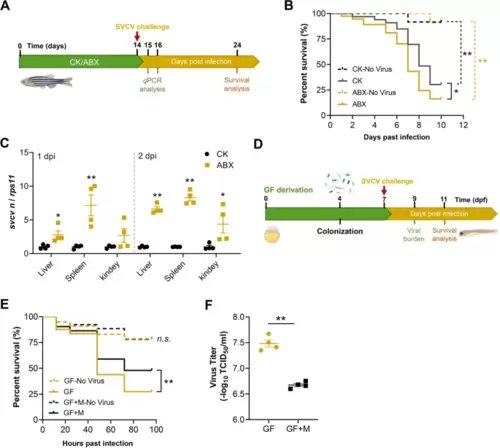
Depletion of intestinal microbiome enhances SVCV infection in zebrafish. A Schematic of the experiment performed in adult zebrafish. B Survival curve of mock or SVCV-infected adult zebrafish fed control or ABX diet (no virus groups: n = 11–12; SVCV groups: n = 39–40). C Viral replication in the liver, spleen, and kidney of SVCV-infected adult zebrafish fed control or ABX diet (n = 4, pool of 6 fish per sample). D Schematic representation for gnotobiotic zebrafish experiment. E Survival curve of mock or SVCV-infected GF and conventionalized zebrafish (n = 80). F Virus titer in GF and conventionalized zebrafish (n = 4, pool of 30 zebrafish larvae per sample). GF, germ-free zebrafish; GF + M, conventionalized zebrafish. B and E log-rank test; C and F unpaired t-test. *p < 0.05, **p < 0.01, n.s., not significant |
|
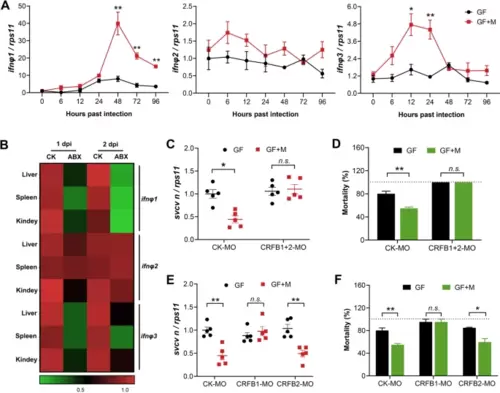
The protective effect of intestinal microbiome against viral infection depends on type I IFN signaling. A Expression of IFNΦ1, IFNΦ2, and IFNΦ3 in SVCV-infected GF or conventionalized zebrafish at different time points (n = 3, pool of 30 zebrafish larvae per sample). B Expression of IFNΦ1, IFNΦ2, and IFNΦ3 in the liver, spleen, and kidney of adult zebrafish after 1 and 2 days post SVCV infection (n = 4, pool of 6 fish per sample). C–D Effect of morpholino-mediated knockdown of type I IFN receptors on SVCV infection. GF and conventionalized zebrafish were treated with control morpholino (CK-MO) or a mixture of CRFB1 and CRFB2 morpholino (CRFB1 + 2 MO) and subjected to SVCV infection. C Viral replication at 48 hpi (n = 5, pool of 30 zebrafish larvae per sample). D Mortality at 96 hpi (n = 3). E–F Effect of morpholino-mediated knockdown of group I or II type I IFN signaling on SVCV infection. GF and conventionalized zebrafish were treated with control morpholino (CK-MO), CRFB1 morpholino (CRFB1-MO), or CRFB2 morpholino (CRFB2-MO) and subjected to SVCV infection. E Viral replication at 48 hpi (n = 5, pool of 30 zebrafish larvae per sample). F Mortality at 96 hpi (n = 3). A, C–F Unpaired t-test. *p < 0.05, **p < 0.01, n.s., not significant |
|
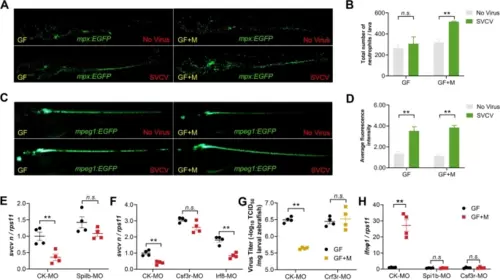
Neutrophil responses to SVCV infection are impaired in the absence of intestinal microbiome. Neutrophils (A) and macrophages (C) were imaged in mock or SVCV-infected GF or conventionalized transgenic zebrafish at 48 hpi. Scale bar, 500 μm. The number of neutrophils (B) and macrophages (D) were analyzed (n = 3). Effect of myeloid cell depletion (Spi1b MO) (E) or selective depletion of neutrophils (Csf3r MO) or macrophages (Irf8 MO) (F) on viral replication in GF or conventionalized zebrafish at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). G Effect of neutrophils depletion (Csf3r MO) on virus titer in GF or conventionalized zebrafish at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). H Effect of myeloid cell depletion (Spi1b MO) or selective depletion of neutrophils (Csf3r MO) on IFNΦ1 expression in GF or conventionalized zebrafish at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). B, D, E–H Unpaired t-test. *p < 0.05, **p < 0.01, n.s., not significant |
|
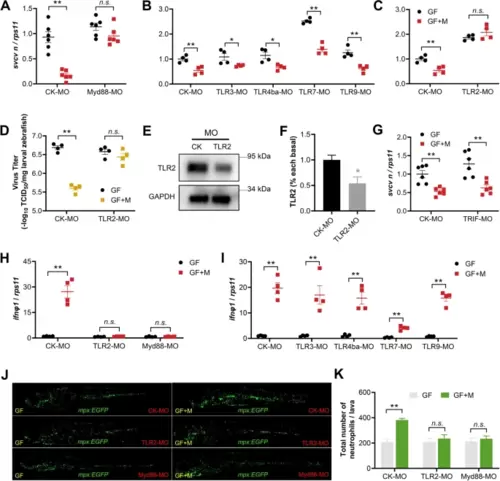
The antiviral effect of intestinal microbiota requires TLR2 and Myd88 signaling. A Effect of Myd88 knockdown on SVCV infection in GF or conventionalized zebrafish at 48 hpi (n = 6, pool of 30 zebrafish larvae per sample). B Effect of morpholino-mediated knockdown of TLR3, TLR4ba, TLR7, and TLR9 on SVCV infection in GF or conventionalized zebrafish at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). C–D Effect of TLR2 knockdown on SVCV infection in GF or conventionalized zebrafish at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). E–F TLR2 morpholino diminished TLR2 protein expression in zebrafish larvae (n = 3, pool of 30 zebrafish larvae per sample). G Effect of TRIF knockdown on SVCV infection in GF or conventionalized zebrafish at 48 hpi (n = 6, pool of 30 zebrafish larvae per sample). Effect of morpholino-mediated knockdown of TLR2, Myd88 (H), TLR3, TLR4ba, TLR7, and TLR9 (I) on IFNΦ1 expression in GF or conventionalized zebrafish at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). J–K Effect of morpholino-mediated knockdown of TLR2 and Myd88 on neutrophil response in GF or conventionalized Tg (mpx:EGFP) zebrafish at 48 hpi. J Confocal imaging of SVCV-infected Tg (mpx:EGFP) zebrafish. Scale bar, 500 μm. K Neutrophil numbers (n = 3). A–D, F–I, K Unpaired t-test. *p < 0.05, **p < 0.01, n.s., not significant |
|
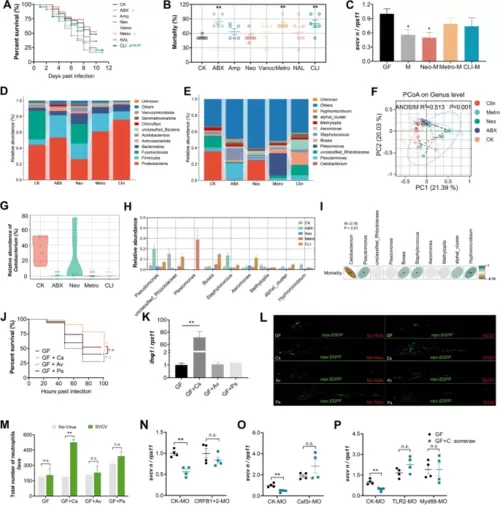
C. somerae recapitulates the antiviral effect of intestinal microbiome. A–B Effect of antibiotics cocktail or single antibiotic feeding on SVCV infection in adult zebrafish. A Survival curve (n = 25). B Mortality (n = 6). C Viral replication in GF zebrafish or GF zebrafish colonized with microbiota derived from adult zebrafish fed with control or antibiotic(s) diet (n = 3, pool of 30 zebrafish larvae per sample). Mock, GF group; M, control microbiota; Neo-M, microbiota from zebrafish fed neomycin; Metro-M, microbiota from zebrafish fed metronidazole; CLI-M, microbiota from zebrafish fed clindamycin. The composition of intestinal microbiota of adult zebrafish fed control or antibiotic(s) diet at phylum (D) and genus level (E) (n = 6, pool of 6 zebrafish per sample). F Principal coordinate analysis (PCoA) of all samples by weighted UniFrac distance at the genus level (n = 6, pool of 6 fish per sample). G The relative abundance of Cetobacterium in intestinal microbiota of adult zebrafish fed control or antibiotic(s) diet (n = 6, pool of 6 zebrafish per sample). H The relative abundance of specific taxa at genus level among groups. I Correlation analysis between the mortality of zebrafish and the relative abundance of genuses (n = 6, pool of 6 fish per sample). J Survival curve of GF zebrafish or GF zebrafish mono-colonized with C. somerae (GF + CS), Aeromonas veronii (GF + AV), or Plesiomonas shigelloides (GF + PS) following SVCV infection (n = 60). K IFNΦ1 expression of GF zebrafish or GF zebrafish mono-colonized with indicated commensal bacterium. Expression was detected at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). L–M Neutrophils response in mock or SVCV-infected GF Tg (mpx:EGFP) zebrafish or GF counterparts mono-colonized with indicated commensal bacterium. L Confocal imaging of transgenic zebrafish. M Neutrophil numbers (n = 3). Samples were collected for imaging at 48 hpi. Scale bar, 500 μm. Effect of type I IFN receptors knockdown (CRFB1 + CRFB2 MO) (N), depletion of neutrophils (Csf3r MO) (O), and TLR2 and Myd88 knockdown (P) on SVCV infection in GF zebrafish or GF counterparts mono-colonized with C. somerae (n = 4, pool of 30 zebrafish larvae per sample). Viral replication was detected at 48 hpi. A and J log-rank test; B, C, G, H, K one-way ANOVA followed by Dunnett’s multiple comparisons test; M–P unpaired t-test. *p < 0.05, **p < 0.01, n.s., not significant |
|
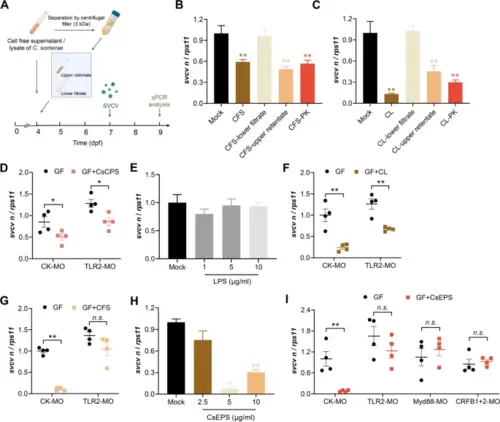
Exopolysaccharides of C. somerae signal through TLR2 to inhibit SVCV infection. A Schematic representation of the study design. B–C Nonprotein macromolecule(s) mediated the antiviral effect of C. somerae. The C. somerae culture suspension was separated into cell free supernatant (CFS) and bacterial cells by centrifugation. CFS and cell lysate (CL) were separated by a 3-kDa filter, or treated with proteinase K. GF zebrafish were treated with different CFS or CL samples and subjected to SVCV infection. Viral replication was detected at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). CFS, cell free supernatant; CL, cell lysate; “-lower filtrate”, 3 kDa filtrate; “-upper retentate”, 3 kDa upper retentate; “-PK”, proteinase K treated CFS or CL samples. D Effect of morpholino-mediated TLR2 knockdown on the antiviral effect mediated by C. somerae capsular polysaccharides (CsCPS) in GF zebrafish. Viral replication was detected at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). E LPS of C. somerae does not have antiviral activity. LPS was extracted by using a commercial kit (Genmed Scientifics Inc., USA). GF zebrafish were treated with different doses of C. somerae LPS and subjected to SVCV infection. Viral replication was detected at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). Effect of morpholino-mediated TLR2 knockdown on the antiviral effect mediated by C. somerae CL (F) or CFS (G) in GF zebrafish. Viral replication was detected at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). H C. somerae exopolysaccharides (CsEPS) inhibited SVCV infection in GF zebrafish. GF zebrafish were treated with different doses of CsEPS and subjected to SVCV infection. Viral replication was detected at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). I Effect of morpholino-mediated knockdown of TLR2, Myd88, and type I IFN receptors on the antiviral effect of CsEPS in GF zebrafish. GF zebrafish were treated with CsEPS at 5 μg/mL and subjected to SVCV infection. Viral replication was detected at 48 hpi (n = 4, pool of 30 zebrafish larvae per sample). B, C, E, H One-way ANOVA followed by Dunnett’s multiple comparisons test; D, F, G, I unpaired t-test. *p < 0.05, **p < 0.01, n.s., not significant |
|
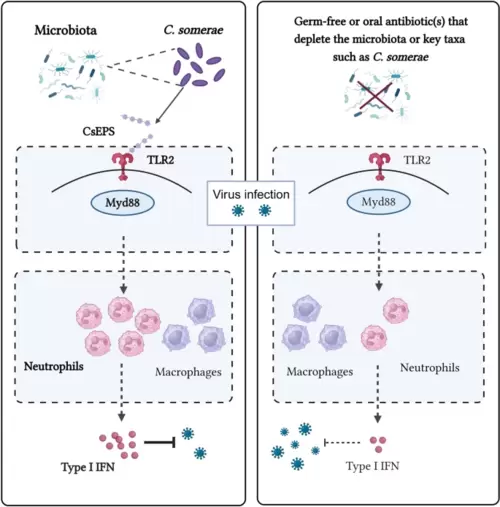
The intestinal microbiome and commensal C. somerae restrict viral infection in zebrafish by priming type I IFN responses in neutrophils through a CsEPS-TLR2-dependent pathway |