- Title
-
Gdf11 regulates left-right asymmetry development through TGF-β signal
- Authors
- Yao, W., Wei, Z., Tian, X., Tan, J., Liu, J.
- Source
- Full text @ Cell Prolif.
|
|
|
|
|
|
|
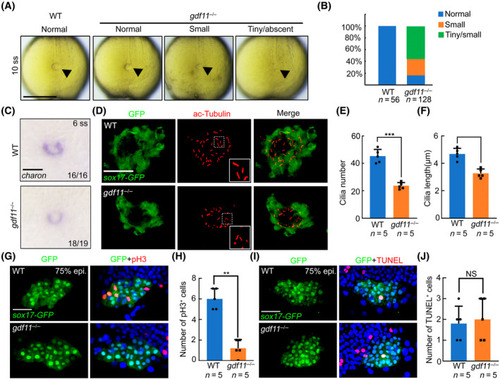
Gdf11 forms heterodimer with Spaw and regulates DFC specification through TGF‐β signal. (A) Immunoprecipitation between zGdf11‐Flag and zSpaw‐Myc in HEK 293T cells. (B) Western blot results of overexpressed zGdf11‐Flag and zSpaw‐Myc in HEK 293T cells with or without SB431542 treatment. (C) |
|
Mechanism diagram of Gdf11 regulating left–right asymmetry development. Gdf11 forms a heterodimer with Spaw, activating the activin receptor and phosphorylating Smad2/3. This process DFC proliferation, subsequently activates the expression of |