- Title
-
Transgenic zebrafish as a model for investigating diabetic peripheral neuropathy: investigation of the role of insulin signaling
- Authors
- Lee, D.W., Park, H.C., Kim, D.H.
- Source
- Full text @ Front. Cell. Neurosci.
|
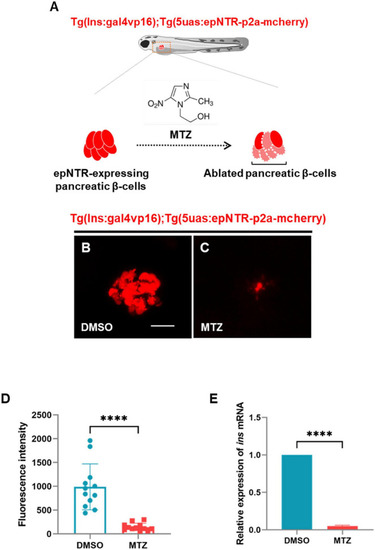
Ablation of pancreatic β-cells induced depletion of insulin production in ins:epNTR-mCherry zebrafish. |
|
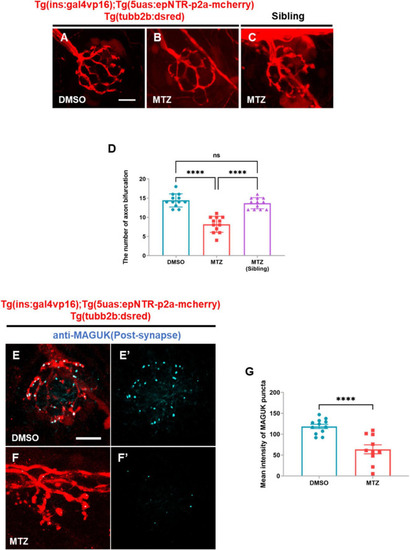
Depletion of insulin production caused peripheral neuropathy in the peripheral lateral line nerve of ins:epNTR-mCherry zebrafish. |
|
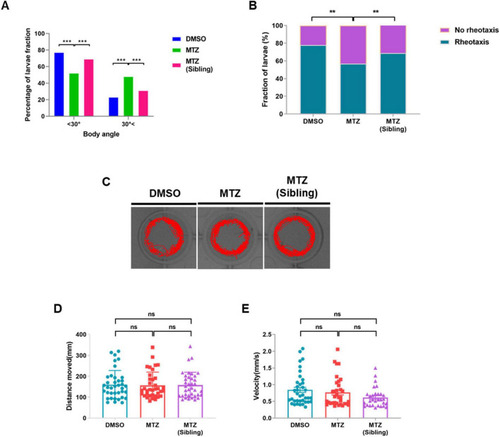
Depletion of insulin production results in defective rheotaxis behavior, but not locomotor behavior, in ins:epNTR-mCherry zebrafish. |
|
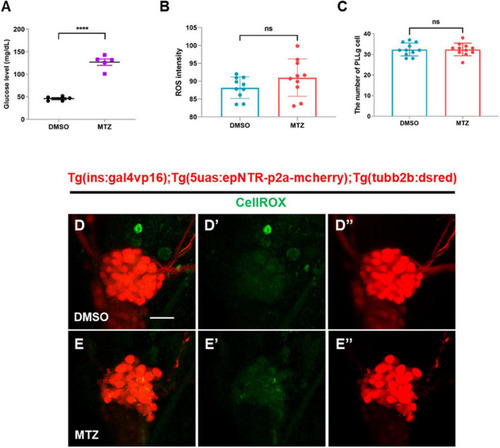
Depletion of insulin production elevates glucose levels, but not ROS, in ins:epNTR-mCherry zebrafish. |
|
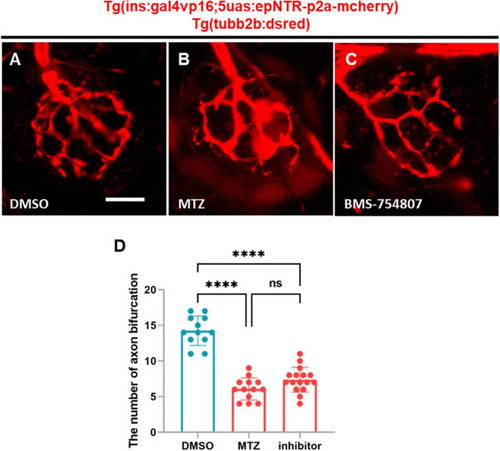
BMS-754807-induced IR inhibition reduces PLL nerve endings in ins:epNTR-mCherry zebrafish. |
|
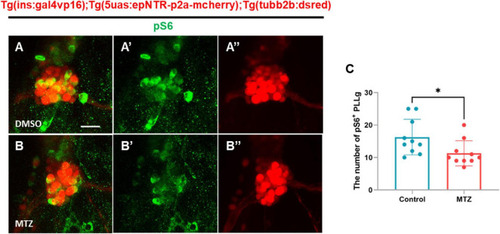
Depletion of insulin production downregulates the mTOR signaling pathway in the PLLg in ins:epNTR-mCherry zebrafish. |