- Title
-
Mutations in the Bone Morphogenetic Protein signaling pathway sensitize zebrafish and humans to ethanol-induced jaw malformations
- Authors
- Klem, J.R., Schwantes-An, T.H., Abreu, M., Suttie, M., Gray, R., Vo, H., Conley, G., Foroud, T.M., Wetherill, L., CIFASD, Lovely, C.B.
- Source
- Full text @ Dis. Model. Mech.
|
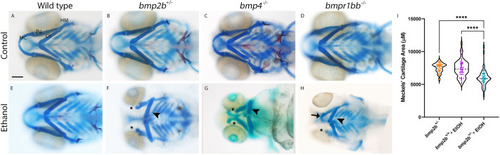
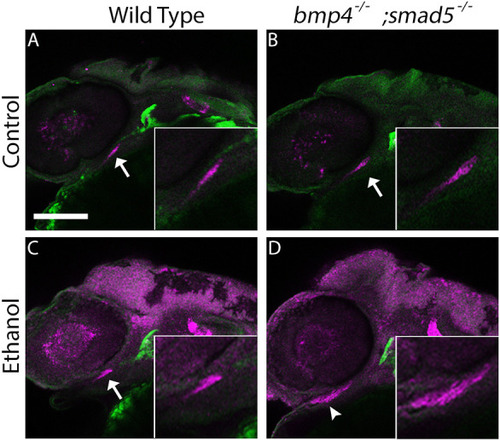
PHENOTYPE:
|
|
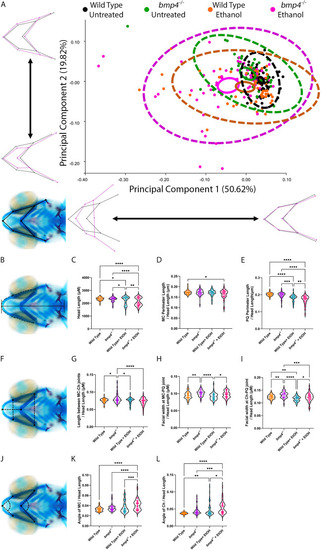
PHENOTYPE:
|
|
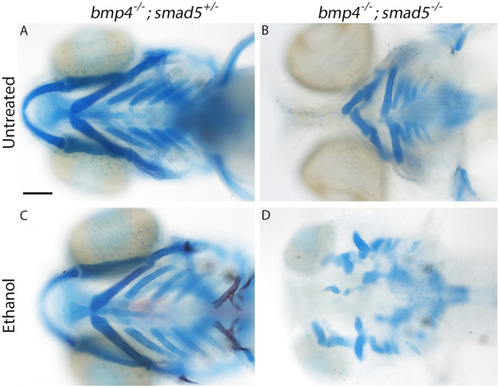
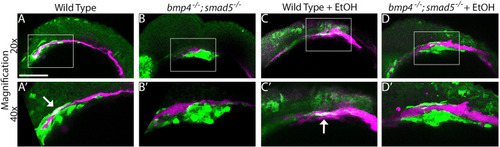
PHENOTYPE:
|
|
|
|
|
|
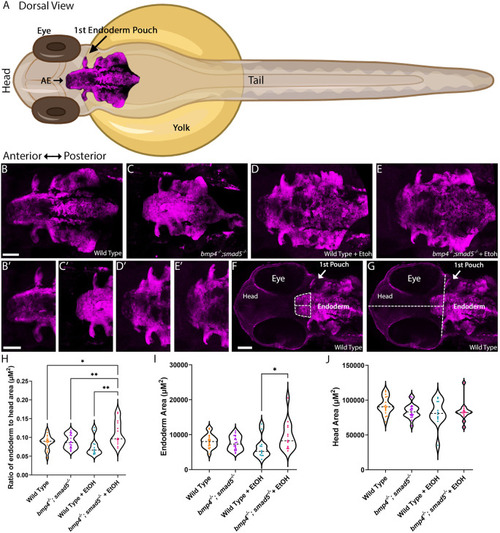
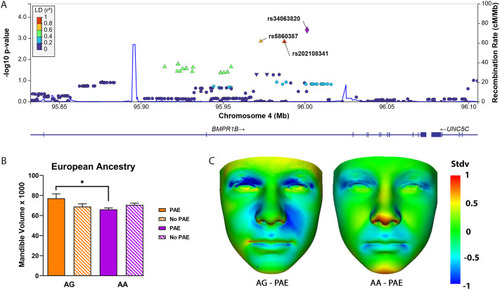
PHENOTYPE:
|
|
|