- Title
-
Piggyback knockdown screening of unique genes of zebrafish young thrombocytes identifies eight novel genes in thrombopoiesis
- Authors
- Fallatah, W., Mary, J., Dhinoja, S., Vallabhaneni, S., Jagadeeswaran, P.
- Source
- Full text @ Sci. Rep.
|
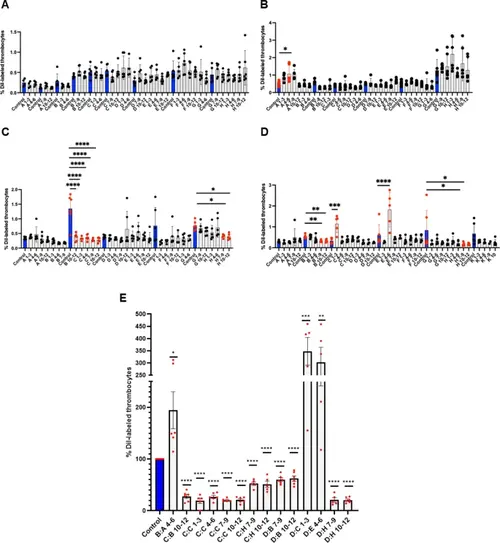
Quantification of DiI-labeled young and mature thrombocytes from flow cytometric data in whole blood after knockdown (KD) of sets of three genes in zebrafish. 394 protein-encoded genes uniquely expressed in RFP + young thrombocytes in adult zebrafish were screened as 132 sets, and DiI-labeled thrombocyte percentage was estimated (A) from set 1 to set 32 (B) from set 33 to set 64 (C) from set 65 to set 96 (D) from set 97 to set 132. (E) the positives from A-D are replotted for easy visualization using a single wild-type (WT) control represented by a blue bar, while all KDs are shown as grey bars to the right of the WT control. Since A-D positives had daily controls, each control value was taken as 100% and the experimental values were normalized to these control percentages and plotted. 4 to 6 fish were utilized for each KD set and controls (N = 4–6). The lines above the bars indicate a significant difference between WT and KD sample sets. One-way ANOVA was used for statistical analysis. Error bars represent mean ± SD. *, **, ***, and **** represent p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, and ≤ 0.0001, respectively. A p-value < 0.05 was considered significant. |
|
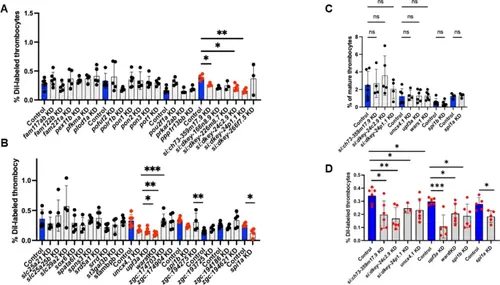
Quantification of young (DiI-labeled) and mature thrombocytes from flow cytometric data in whole blood after individual gene knockdown in zebrafish. Each of the 42 protein-encoded genes from 14 sets obtained from primary screen was subjected to secondary screening. (A) and (B) DiI-labeled thrombocyte percentages and (C) mature thrombocyte percentages derived from total and DiI-labeled thrombocyte percentages. (D) DiI-labeled thrombocyte percentages of the positives from A and B subjected to knockdown with a different set of ASOs. Daily wild-type (WT) controls are represented by blue bars, while all KDs are depicted as grey bars to the right of the WT daily controls. 4–6 fish were utilized for each KD of the 42 protein-encoded genes and control experiments. The lines above the bars indicate a significant difference between WT and knockdown of each gene. One-way ANOVA was used for statistical analysis. Error bars represent mean ± SD. *, **, and *** represent p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001, respectively. A p-value < 0.05 was considered significant. p > 0.05 was taken as non-significant (ns). |
|
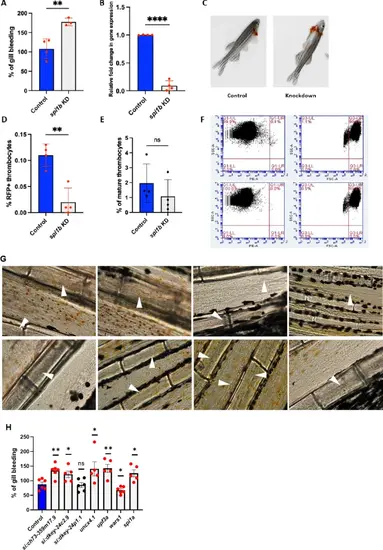
Gill bleeding and quantification of young and mature thrombocytes in spi1b knockdown zebrafish. Gill bleeding in spi1b knockdown fish and confirmation of knockdown by qRT-PCR. (A) Knockdown of spi1b in adult zebrafish and estimation of the extent of bleeding. NaOH-induced gill bleeding was photographed, and the red pixel intensities in the photograph measured the bleeding extent. The percentage of gill bleeding of WT controls is represented by blue bars, while spi1b knockdown is represented by grey bars. 3–4 fish were used for KD sample and control experiments (N = 3–4). (B) The spi1b were knocked down, and the RNA from the liver and spleen were reverse transcribed and subjected to qRT-PCR, and the fold change of gene expression was calculated in wild-type (WT) controls (blue bars) and knockdown (KD) samples (grey bars). Four fish were used for KD and control experiments (N = 4). (C) Representative images showing gill bleeding in wild-type control and the knockdown fish. (D) Quantification of young RFP+ thrombocyte percentages from flow cytometric data in spi1b knockdown adult GloFish whole blood compared to control wild-type (WT) fish. (E) Mature thrombocyte percentages derived from total and RFP + thrombocyte percentages in spi1b knockdown adult GloFish whole blood compared to control WT fish. WT controls are represented by blue bars, while KDs are depicted as grey bars. Four fish were utilized for spi1b KD and control experiments (N = 4). (F) Top and bottom panels show representative dot plots illustrating the percentage of RFP + thrombocytes and the total thrombocytes in whole blood in WT controls and spi1b KD, respectively. The PE-A, SSC-A (Side Scattering), and FSC-A (Forward Scattering) channels are indicated on the respective axes. The Q1-UR in left panels (upper right quadrangle) and Q3-UL in right panels (upper left quadrangle) show the RFP + young and total thrombocyte percentages, respectively. The cells in other quadrangles represent other blood cells. Gating is shown by red lines and is drawn according to the fluorescence intensity of thrombocytes in the PE-A channel and the size in right panel. (G) Representative blood vessels in the tails of WT control (top panels) and spi1b KD (bottom panels) zebrafish, respectively, are shown. Arrowheads show the vessels in the tail region. (H) Knockdown of seven genes in adult zebrafish and estimation of the extent of bleeding. The percentage of gill bleeding in WT control is represented by blue bar, while the knockdown of other genes is represented by grey bars. 5–6 fish were used for KD sample and control experiments (N = 5–6). For A, B, D, E and H, the lines on the top of the bars represent a significant difference between WT and KD samples. Statistical analysis was performed using Student’s t-test. Error bars represent mean ± SD. **, and **** represent p ≤ 0.01 and p ≤ 0.0001, respectively. A p-value < 0.05 was considered significant, and a p > 0.05 was taken as non-significant (ns). |
|
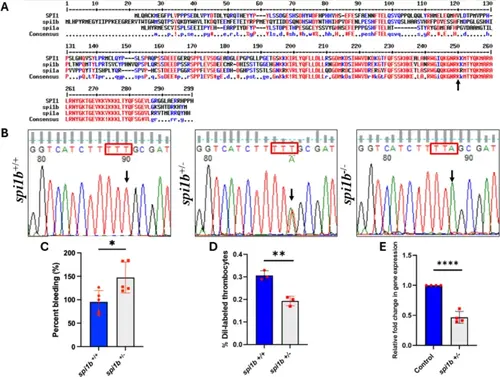
Comparison of Spi1 protein sequences and characterization of spi1b mutant fish. (A) Alignment of the predicted protein sequences of zebrafish Spi1b, Spi1a and human SPI1 transcription factors showing homologies. The amino acids highlighted in red are conserved (high consensus) between zebrafish and humans. Blue indicates low consensus sequences, and black indicates neutral according to the MultAlin program. The black arrow indicates the amino acid lysine replaced by the terminator codon, leading to termination at arginine just before lysine. (B) DNA sequencing of spi1b mutant fish. The DNAs, from adult wild-types (WT) and heterozygotes obtained from the ZIRC were amplified by PCR and resolved on agarose gel electrophoresis. The DNAs from these bands were eluted and sequenced. The heterozygotes were crossed, and the DNA from the progeny 5 dpf larvae was sequenced as described above. Since homozygotes could not be obtained, the heterozygotes were subjected to gill bleeding. Chromatograms of sequences from spi1b +/+, spi1b +/−, and spi1b −/− are shown. Wild-types (WT), heterozygotes and homozygotes had TTT, TTT/TTA, TTA sequences respectively. Note the sequences are in the non-coding strand. (C) Gill bleeding in spi1b +/− mutant fish. In adult spi1b +/− zebrafish, NaOH-induced gill bleeding was photographed, and the red pixel intensities in the photograph measured the bleeding extent. The percentage of gill bleeding of WT controls (spi1b +/+) is represented by blue bars, while spi1b +/− mutant is represented by grey bars. 5 fish were used for KD sample and control experiments (N = 5). (D) DiI-labeled thrombocyte percentages of the WT controls (spi1b +/+) and spi1b +/− mutant. Three fish were used for mutant and control experiments (N = 3). (E) RNA from the liver and spleen from spi1b +/− mutant was reverse transcribed and subjected to qRT-PCR, and the fold change of gene expression was calculated in wild-type controls (blue bars) and spi1b +/− mutant (grey bars). Four fish were used for mutant and control experiments (N = 4). The lines on the top of the bars represent a significant difference between WT and mutant samples. Statistical analysis was performed using Student’s t-test. Error bars represent mean ± SD. *, **, and **** represent p ≤ 0.05, p ≤ 0.01 and p ≤ 0.0001, respectively. |