- Title
-
Cre-Lox miRNA-delivery technology optimized for inducible microRNA and gene-silencing studies in zebrafish
- Authors
- Guo, F., Tromp, A., Wang, H., Hall, T.E., Giacomotto, J.
- Source
- Full text @ Nucleic Acids Res.
|
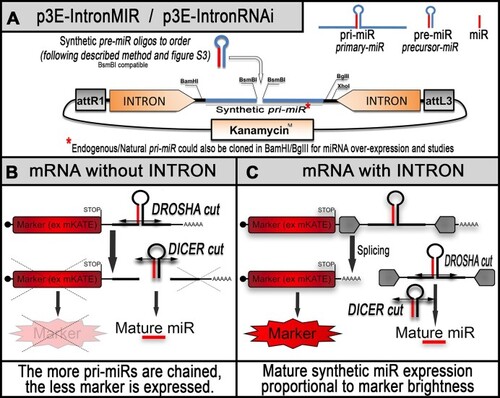
Schematics of the new RNAi backbone for zebrafish gene silencing. ( |
|
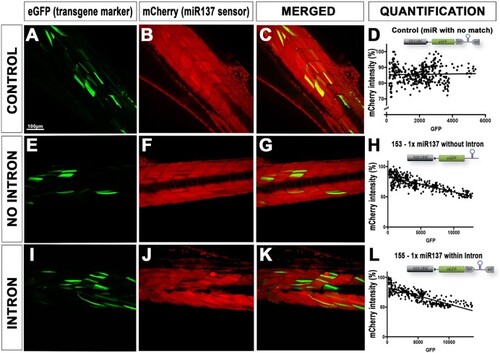
Validating effect of intron based RNAi approach on endogenous miRNA processing. ( |
|
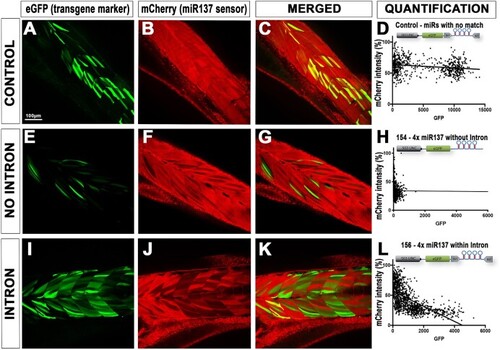
Validation of intron based RNAi approach on rescuing co-expression of fluorescent markers. ( |
|
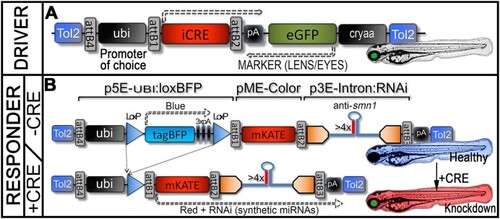
Conditional Cre/Lox RNAi genetic system. ( |
|
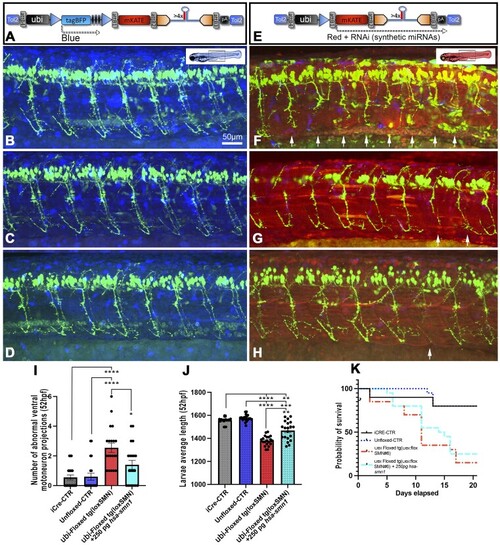
Representative snapshots and phenotypic analysis of 50 hpf zebrafish larvae with unfloxed (A–D) or floxed (E–H) integrated RNAi transgene. ( |
|
Cell-specific miR-delivery/RNAi in zebrafish does not require transposase. We tested the ability of the presented miR-delivery system to enable rapid cell-specific experiments. The approach is similar to the method conducted in Figures |