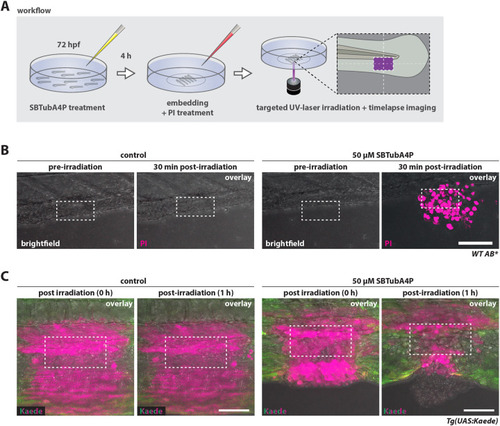
SBTubA4P induces spatially confined cell death in larval zebrafish tissues. (A) Schematic representation of workflow for SBTubA4P-mediated cell ablation in zebrafish larvae in vivo. Zebrafish larvae at 3 days post fertilization (dpf) were incubated with SBTubA4P for 4 h, anesthetized, then washed and embedded in agarose on imaging dishes. Control larvae were not treated with SBTubA4P, but otherwise underwent the same procedure. PI solution was added to the medium before imaging. Targeted illumination was performed by repeated scanning of a defined area using the UV laser diode of a Leica SP8 WLL confocal microscope. hpf, hours post fertilization. (B) Targeted cell ablation through SBTubA4P illumination in live zebrafish tissues. Wild-type (WT) AB zebrafish were treated as outlined in A, targeting an area of 95×47.5 µm (depicted by the dashed line rectangles). PI staining indicates cell death (see Movie 5). Left panel, control zebrafish without SBTubA4P; right panel, zebrafish with SBTubA4P. Representative confocal images from two independent experiments. Scale bar: 100 µm. (C) Embryos of the transgenic line Tg(UAS:Kaede)rk8 were microinjected with 25 pg KalTA4 mRNA at the one-cell stage for ubiquitous Kaede expression. At 3 dpf, larvae were treated with SBTubA4P as outlined in A but without the addition of PI. A rectangular region of interest (ROI) of 95×47.5 µm (depicted by the dashed line rectangles) was UV irradiated in control (left panel) and SBTubA4P (right panel) larvae. Red fluorescence (shown in magenta) indicates the area affected by UV through photoconversion of Kaede (see Movie 6). Representative confocal images (n=3 per condition). Scale bars: 50 µm.
|