Fig. 1
- ID
- ZDB-FIG-241202-6
- Publication
- García-Pupo et al., 2024 - Amylovis-201 is a new dual-target ligand, acting as an anti-amyloidogenic compound and a potent agonist of the σ1 chaperone protein
- Other Figures
- All Figure Page
- Back to All Figure Page
|
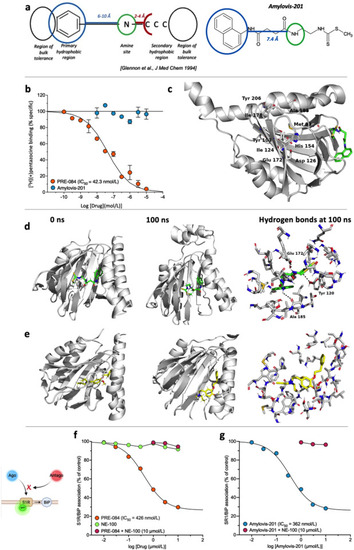
Amylovis-201 is a σ1 receptor interacting drug. (a) Pharmacophore structure proposed by Glennon et al.14 and application to the developed formula of Amylovis-201. Note that the secondary hydrophobic region does not fit with the hydrophilic dithiocarbamate part. (b) Inhibition of (+)-[3H]pentazocine specific binding to Jurkat human leukemic T cells by PRE-084 and Amylovis-201. (c) Amino acids identified in the ligand binding pocket (LBP) of the σ1 protein and which are critical for ligand-protein interactions. In the 3D model of the protein, the LBP is profoundly occluded within the protein and unreachable for Amylovis-201. Molecular dynamics were performed between 0 and 100 ns with (d) Amylovis-201 and (e) NE-100. H-bonds are shown as dashed lines at 100 ns (f) PRE-084 dissociated BiP from σ1 protein in GFP-tagged σ1 protein-overexpressing CHO cells with an IC50 value of 426 nmol/L. NE-100 failed to do so at concentrations up to 10 μmol/L, but, at 10 μmol/L, prevented the dissociating effect of PRE-084. (g) Amylovis-201 dissociated BiP from σ1 protein with an IC50 of 362 nmol/L. Its effect was blocked by NE-100. Experiments were performed in duplicates. |