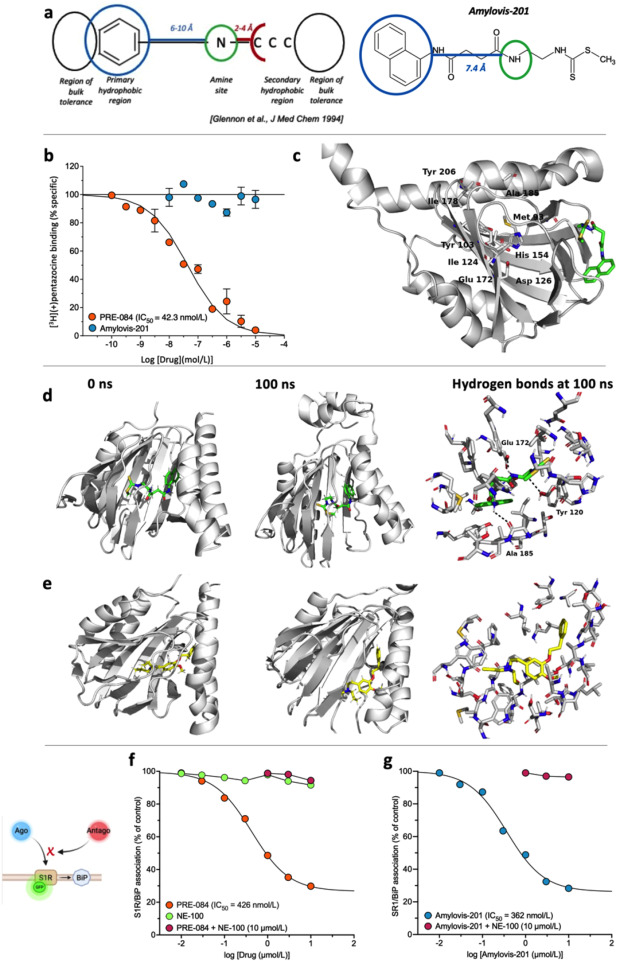
Fig. 1 Amylovis-201 is a σ1 receptor interacting drug. (a) Pharmacophore structure proposed by Glennon et al.14 and application to the developed formula of Amylovis-201. Note that the secondary hydrophobic region does not fit with the hydrophilic dithiocarbamate part. (b) Inhibition of (+)-[3H]pentazocine specific binding to Jurkat human leukemic T cells by PRE-084 and Amylovis-201. (c) Amino acids identified in the ligand binding pocket (LBP) of the σ1 protein and which are critical for ligand-protein interactions. In the 3D model of the protein, the LBP is profoundly occluded within the protein and unreachable for Amylovis-201. Molecular dynamics were performed between 0 and 100 ns with (d) Amylovis-201 and (e) NE-100. H-bonds are shown as dashed lines at 100 ns (f) PRE-084 dissociated BiP from σ1 protein in GFP-tagged σ1 protein-overexpressing CHO cells with an IC50 value of 426 nmol/L. NE-100 failed to do so at concentrations up to 10 μmol/L, but, at 10 μmol/L, prevented the dissociating effect of PRE-084. (g) Amylovis-201 dissociated BiP from σ1 protein with an IC50 of 362 nmol/L. Its effect was blocked by NE-100. Experiments were performed in duplicates.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Acta Pharm Sin B