- Title
-
Olfactory Dysfunction in a Novel Model of Prodromal Parkinson's Disease in Adult Zebrafish
- Authors
- Vorhees, N.W., Groenwold, S.L., Williams, M.T., Putt, L.S., Sanchez-Gama, N., Stalions, G.A., Taylor, G.M., Van Dort, H.E., Calvo-Ochoa, E.
- Source
- Full text @ Int. J. Mol. Sci.
|
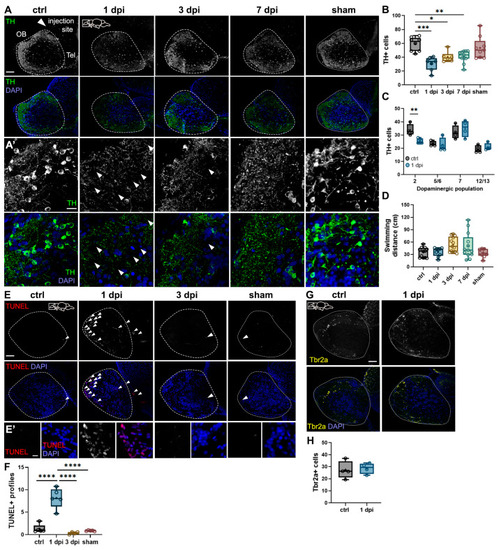
Effects of 6-OHDA injections on the zebrafish brain. ( |
|
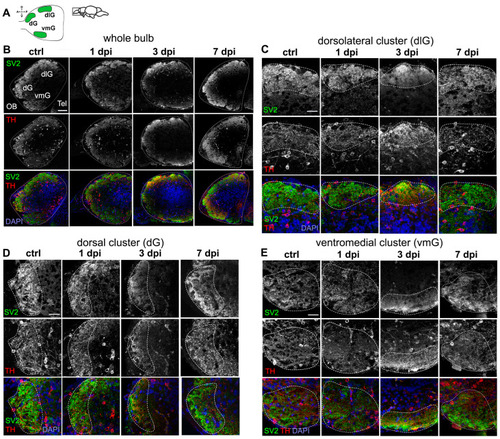
Dysregulation of synaptic contacts in the OB caused by 6-OHDA. ( |
|
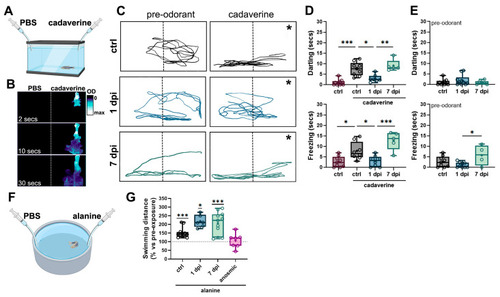
6-OHDA injections selectively reduce olfactory-mediated responses. ( |
|
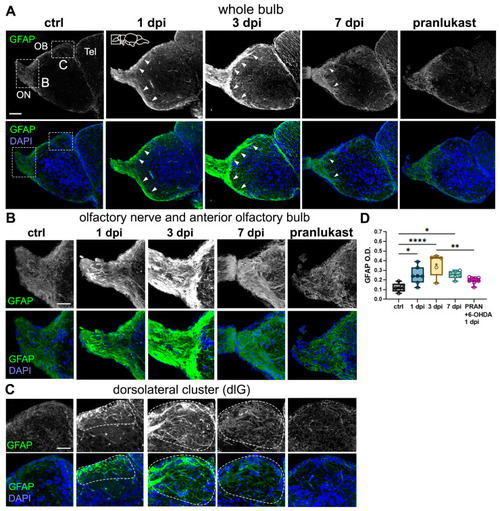
6-OHDA injections lead to astroglial activation in the OB. ( |
|
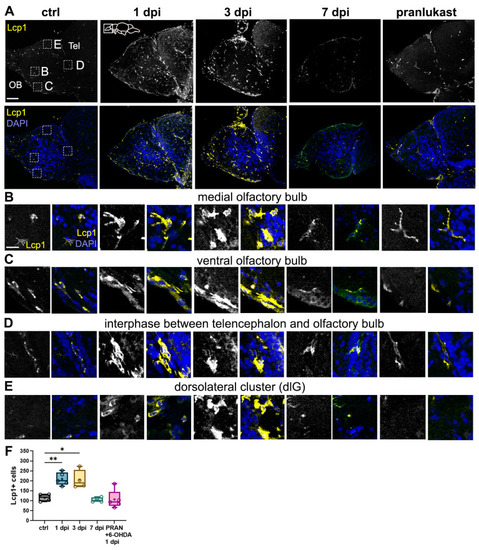
Microglial activation and leukocyte migration in the OB following 6-OHDA injection. ( |
|
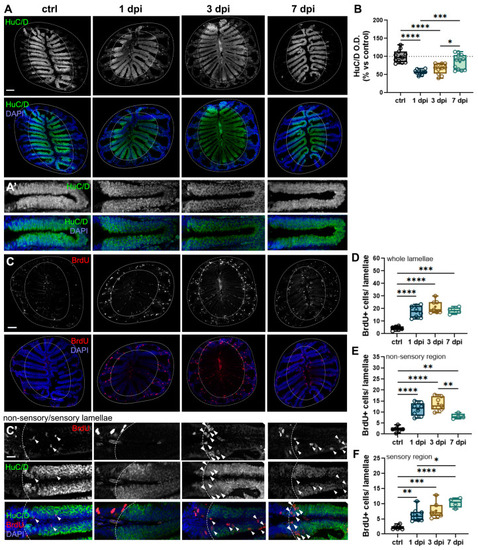
6-OHDA injections in the OB cause retrograde degeneration in the OE. ( |
|
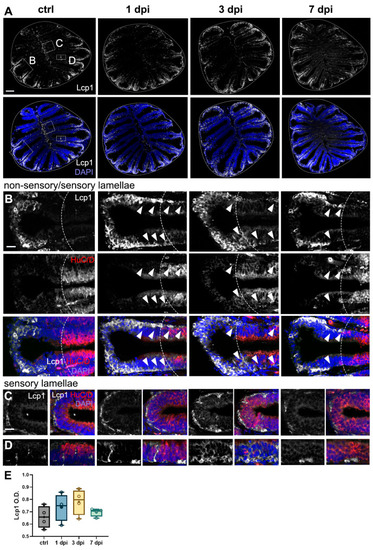
Leukocytic migration within the OE caused by 6-OHDA injections in the OB. ( |