- Title
-
Srebf2 mediates successful optic nerve axon regeneration via the mevalonate synthesis pathway
- Authors
- Hu, M., Veldman, M.B.
- Source
- Full text @ Mol. Neurodegener.
|
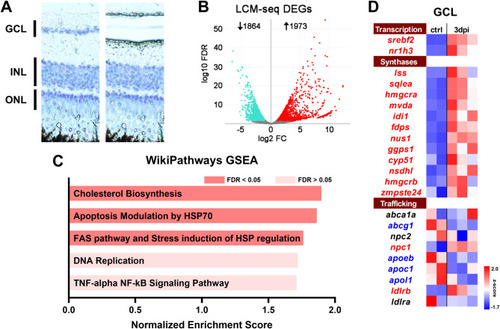
LCM-seq analysis of 3 dpi retinal ganglion cell layer (GCL) identifies upregulation of the cholesterol synthesis pathway. ( |
|
|
|
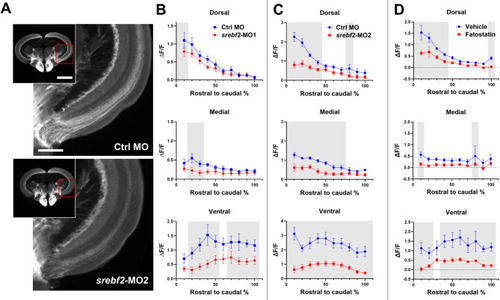
Srebf2 loss-of-function inhibits axon regeneration in the optic tectum at 7 dpi. ( |
|
Srebf2 loss-of-function delays dorsal light response (DLR) recovery. ( |
|
Conditional expression of constitutively active |
|
The Cholesterol Biosynthesis pathway is coordinately regulated in the retinal GCL by |
|
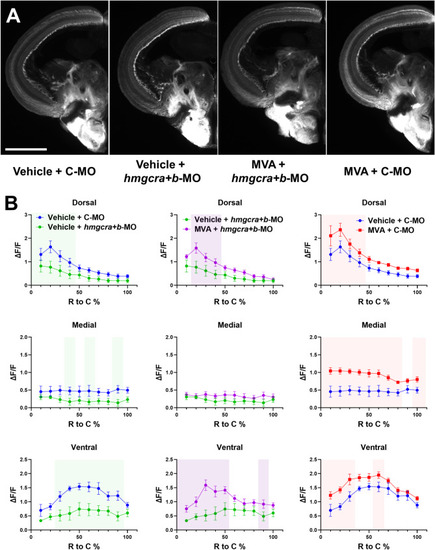
GCL mev/chol synthesis genes are dependent on |
|
Axon regeneration into the optic tectum is dependent upon the mevalonate synthesis pathway. ( |