- Title
-
Elevated Serum Homocysteine Levels Impair Embryonic Neurodevelopment by Dysregulating the Heat Shock Proteins
- Authors
- Mai, J., Yang, L., Wang, M., Deng, J.M., Min, M., Xie, H.J., Jiang, Y.M., Sun, H.Q., Liu, X.J.
- Source
- Full text @ Dev. Neurobiol.
|
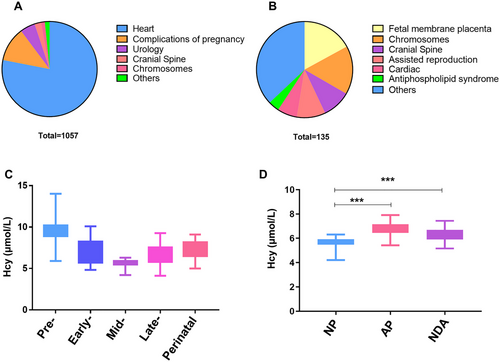
Higher serum Hcy levels was associated with abnormal fetal neurodevelopment: (A) proportion of various abnormal causes with adverse pregnancy outcomes of live births; (B) proportion of various abnormal causes with failure to deliver a viable newborn; (C) distribution of serum Hcy levels in pre-, early-, mid-, late-pregnancy and perinatal period of normal pregnant women; (D) comparison of serum Hcy levels in mid- pregnancy among pregnant women with normal pregnancy (NP) outcomes, adverse pregnancy (AP) outcomes, and neurodevelopmental abnormalities (NDA) outcomes among them; ***p < 0.001. |
|
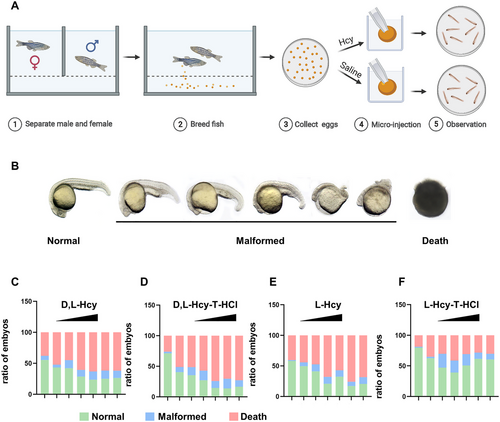
High Hcy induce neurodevelopmental abnormalities in zebrafish embryos: (A) the flow diagram of Hcy exposure of zebrafish embryos, (B) four forms of Hcy treatment of zebrafish embryos (< 2 hpf) produced malformed phenotypes with progressively increasing severity of malformations; (C–F) the proportion of normal, deformed and dead embryos in each group observed at 24 hpf after without injection and with concentration at 0, 100, 200, 500, 1000, 2000, and 4000 µm of four Hcy forms were treated with zebrafish embryos. |
|
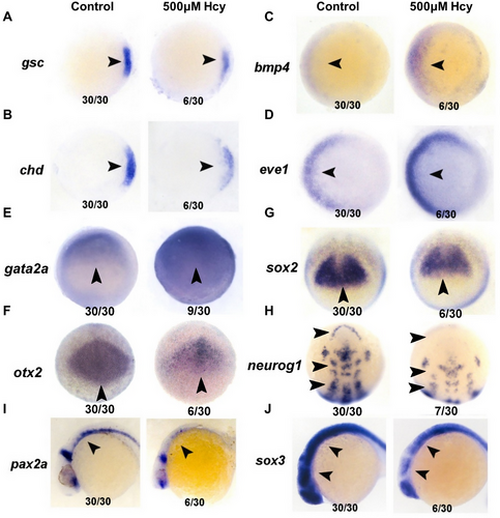
Hcy treatment results in markedly abnormal expression of genes related to body axis and neurodevelopment in zebrafish embryos: (A–E) the developmental stage at which the marker genes gsc, chd, bmp4, eve1, and gata2a were detected was 6 hpf; (F and G) sox2, otx2 were detected at 7 hpf; (H) Neurog1 was detected at 10 hpf; (I and J) sox3 and pax2a were detected at 24 hpf. The arrows in the figure indicate the position of the gene with differential expression on the embryo, the numbers below on the left indicate the number of embryos of that type, and the numbers on the right indicate the total number of embryos.Hcy treatment results in markedly abnormal expression of genes related to body axis and neurodevelopment in zebrafish embryos: (A–E) the developmental stage at which the marker genes gsc, chd, bmp4, eve1, and gata2a were detected was 6 hpf; (F and G) sox2, otx2 were detected at 7 hpf; (H) Neurog1 was detected at 10 hpf; (I and J) sox3 and pax2a were detected at 24 hpf. The arrows in the figure indicate the position of the gene with differential expression on the embryo, the numbers below on the left indicate the number of embryos of that type, and the numbers on the right indicate the total number of embryos. |
|
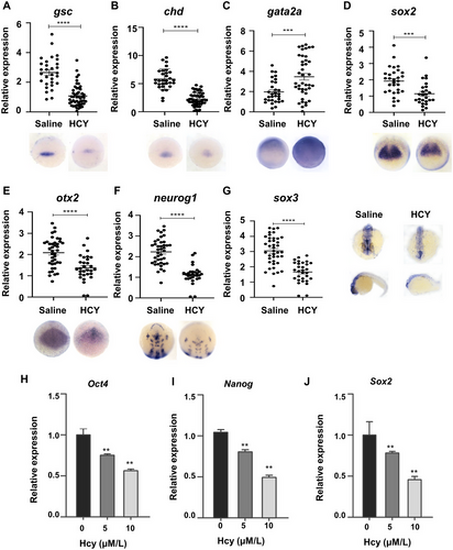
Hcy treatment results in markedly abnormal expression levels of key genes in zebrafish embryonic body axis and neurodevelopment: (A and B) dorsal tissue center marker gene; (C) epidermal ectoderm marker genes; (D and E) neural precursor cell and neural plate marker genes; (F) neural plate marker gene; (G) central nervous system marker genes; ***p < 0.001; ****p < 0.0001, (H–J) real-time qPCR analysis of oct4, sox2, and nanog of Hcy treatment. |
|
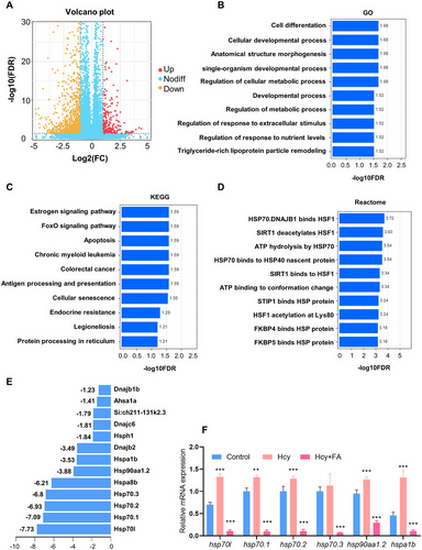
Hcy regulate the expression of HSP genes in zebrafish embryos: (A) representative volcano of the DEGs; (B) Top 10 enriched in GO analysis of the DEGs; (C) Top 10 enriched in KEGG analysis of the DEGs; (D) Top 10 enriched in Reactome analysis of the DEGs; (E) expression of HSP-related genes of Hcy treatment; and (F) RT-qPCR results showed that Hcy treatment resulted in significant upregulation of major HSP-related genes and folic acid can inhibit this increase. |
|
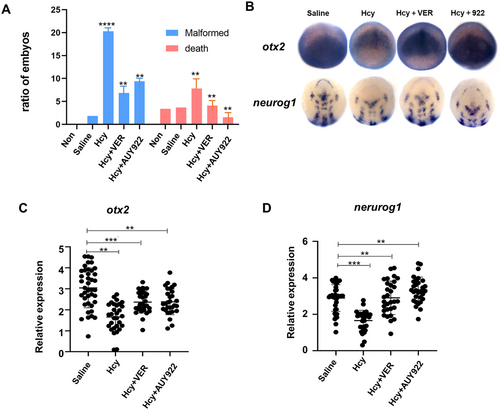
HSP70/90 inhibitor markedly restores neurodevelopmental malformation caused by Hcy: (A) the mortality and deformity rates of each group; (B) WISH analysis of otx2 and neurog1 in different treatment, and (C and D) the expression levels of neural progenitor cell marker gene otx2 and neural plate marker gene neurog1 in each group were analyzed in the recovery experiment. |