- Title
-
Carbonic anhydrase inhibition ameliorates tau toxicity via enhanced tau secretion
- Authors
- Lopez, A., Siddiqi, F.H., Villeneuve, J., Ureshino, R.P., Jeon, H.Y., Koulousakis, P., Keeling, S., McEwan, W.A., Fleming, A., Rubinsztein, D.C.
- Source
- Full text @ Nat. Chem. Biol.
|
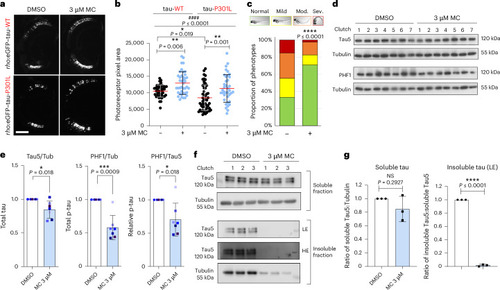
Methocarbamol ameliorates tau-induced toxicity and reduces hyperphosphorylated and insoluble tau levels in zebrafish models. |
|
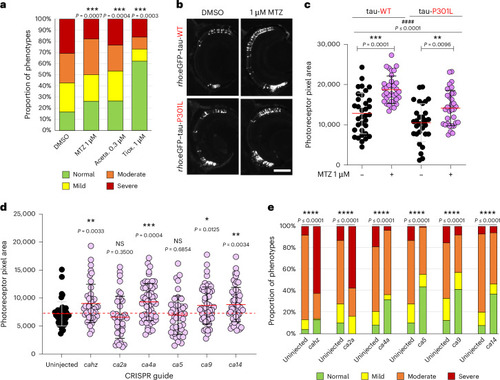
The rescuing effect of methocarbamol relies on its primary pharmacological target, the CA family. |
|
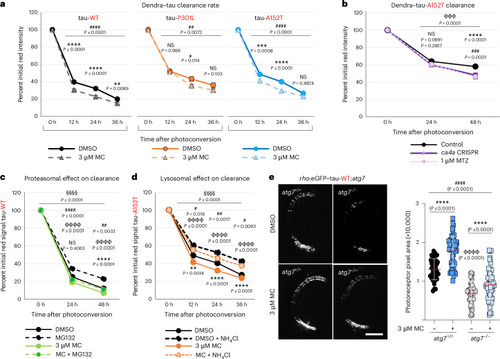
CA inhibition increases the clearance rate of tau without affecting proteasomal or autophagic degradation in vivo. |
|
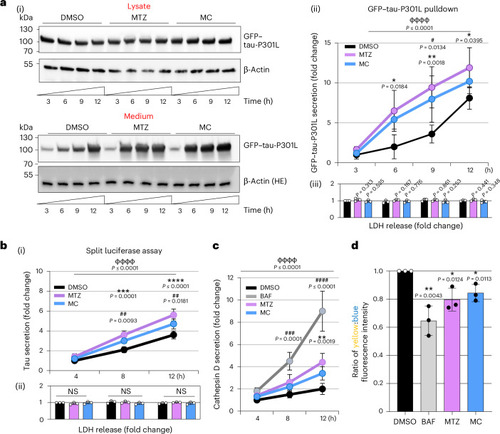
CA inhibition induces the secretion of tau via lysosomal exocytosis. |
|
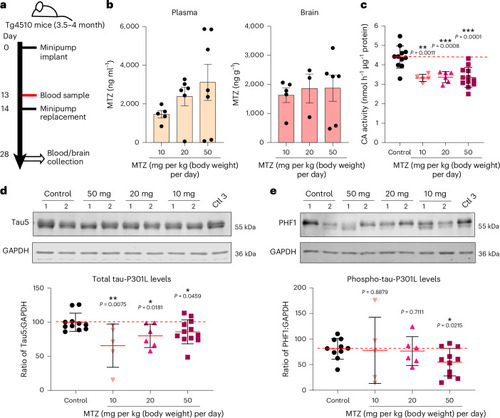
CA inhibition reduces total and phospho-tau levels in Tg4510 tau transgenic mice. |
|
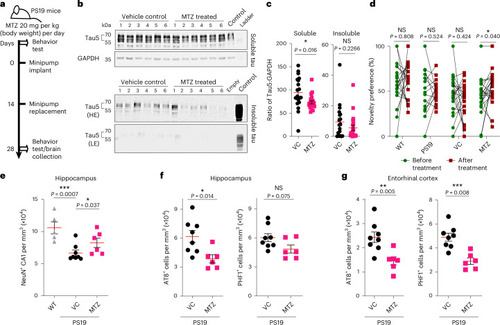
Methazolamide treatment reduces tau levels and neuron loss and improves object recognition in PS19 tau transgenic mice. |