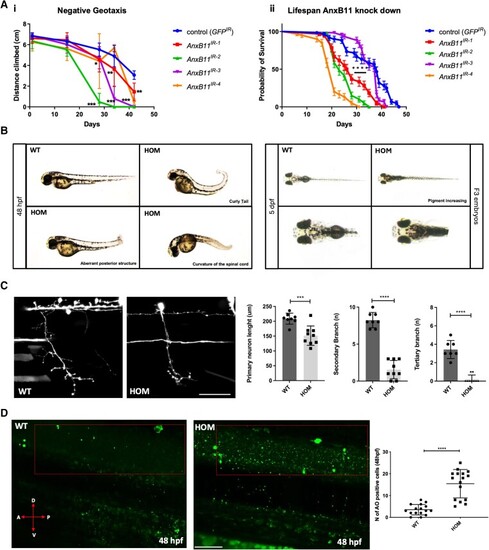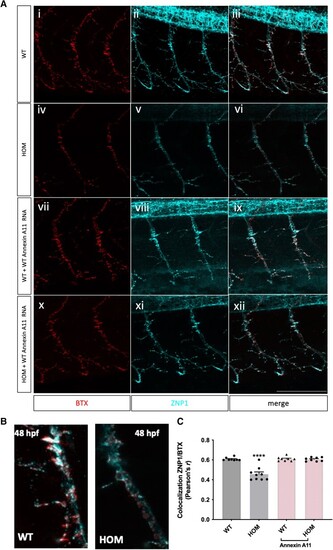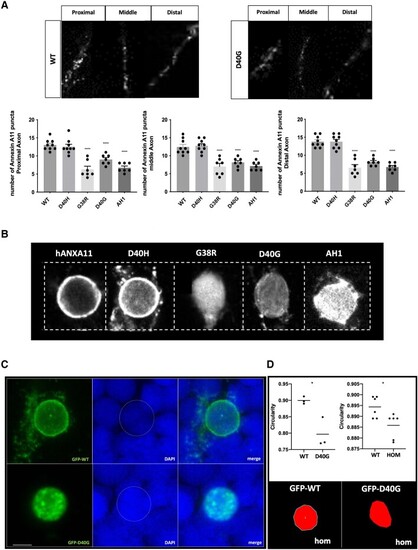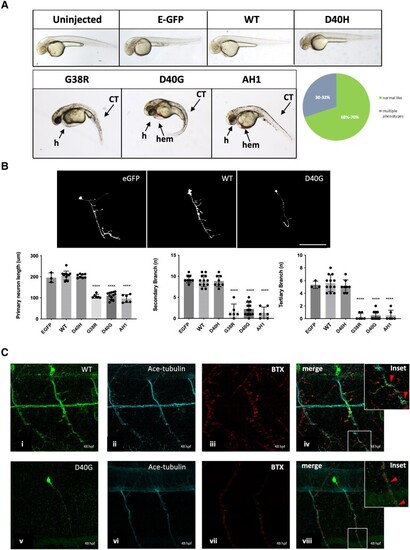
Misexpression of p.D40G, p.G38R and AH1-Annexin A11 in AB Zebrafish result in severe morphological defects at 48 hpf. (A) Lateral view of AB larvae injected at the one-cell stage expressing MNX1:GAL4 and a mix of either empty-UAS GFP, or human Annexin A11-GFP plasmids of wild-type (WT), the non-disease control D40H and mutants D40G, G38R and AH1 (alpha helical 1 deletion) and imaged at 48 hours post-fertilization (hpf). Approximately 30% of larvae with G38R, D40G and AH1 mutants showed a range of morphological phenotypes i.e. curly tail (CT), haemorrhage (hem) and heart defects (h) (n = 40 each group, n = 12 G38R, D40G and AH1 deformed embryos each time, four biological replicates). (B) Live imaging of caudal primary (CaP) motor neurons from normal like GFP positive (+ve) larvae (free of morphological abnormalities) showing representative images (left to right) injected with empty vector-eGFP, wild-type Annexin A11-eGFP and D40G-Annexin A11-eGFP. Neurons imaged from sagittal and cross-sectional view show a single neuron innvervated by a cap neuron (top). Quantitative analysis of axonal length and branching after microinjection with control eGFP vector, wild-type Annexin A11 and amyotrophic lateral sclerosis (ALS) and AH1 mutants (bottom). Statistical analysis was performed on GraphPad Prism 7 using a one-way ANOVA with a post hoc Tukey’s multiple comparisons test. ****P ≤ 0.0001, n = 3: eGFP: n = 12 WT-Annexin A11-eGFP, n = 12: D40G- n = 6 G38R and n = 6 AH1 Annexin A11-eGFP (one axon per biological replicate, maximum projection). Scale bar = 100μm. (C) The D40G mutation results in a reduced amount of axonal Annexin A11 expression and clustering with acetylcholine receptors. [C(i–viii)] Confocal images of zebrafish trunk, lateral view, anterior to the right, imaged at 48 hours post-fertilization (hpf) after microinjection with WT-Annexin A11-eGFP and D40G-Annexin A11-eGFP mutant plasmids and followed by GFP [C(i and v)], ac-Tubulin [C(ii and vi, cyan)] and staining BTX [C(iii and vii, red)]. GFP immunohistochemistry (IHC) demonstrates decreased or absent branching of motor axons in D40G injected embryos, compared to embryos injected with WT-Annexin A11. Insets on [C(iv and viii)] represent effects on postsynaptic development (red arrows and white square). Max projections. Scale bar = 100μm. eGFP = enhanced green fluorescent protein.
|