- Title
-
Decoding the molecular, cellular, and functional heterogeneity of zebrafish intracardiac nervous system
- Authors
- Pedroni, A., Yilmaz, E., Del Vecchio, L., Bhattarai, P., Vidal, I.T., Dai, Y.E., Koutsogiannis, K., Kizil, C., Ampatzis, K.
- Source
- Full text @ Nat. Commun.
|
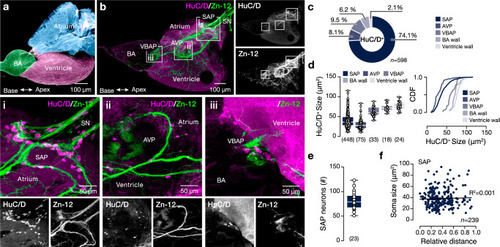
Neuroanatomy of the adult zebrafish intracardiac nervous system. |
|
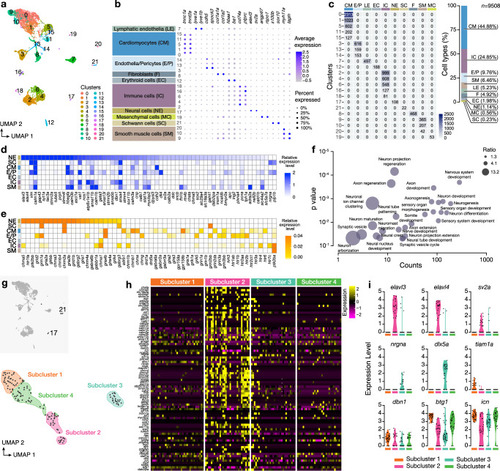
Single-cell transcriptomics from the adult zebrafish heart. |
|
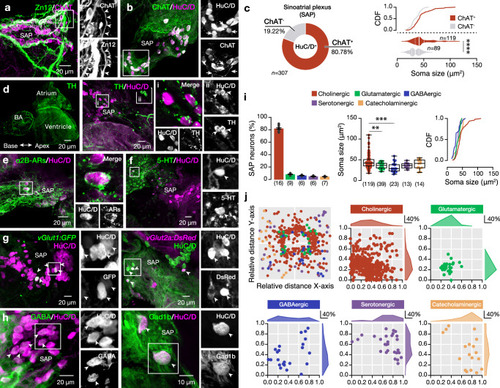
Neurochemical variability in the intracardiac SAP neuronal population. |
|
Diverse cellular properties of the intracardiac SAP neurons. |
|
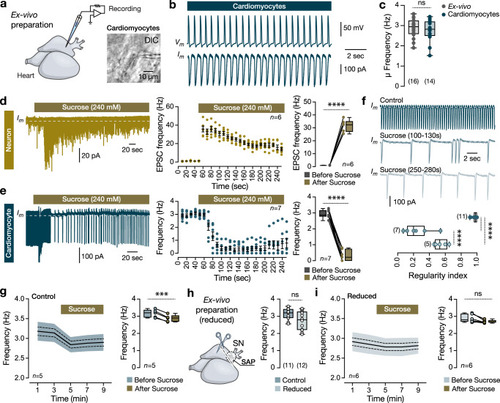
Functional changes in heart rate. |