- Title
-
Semaphorin 3F is elevated in serum of heart failure patients and inhibits cardiac angiogenesis via the VEGF/Akt/eNOS pathway
- Authors
- Petrova, D., Weberbauer, M., Reichert, S., Scheid, S., Esser, J., Fink, K., Duerschmied, D., Moser, M., Helbing, T.
- Source
- Full text @ J Mol Cell Cardiol Plus
|
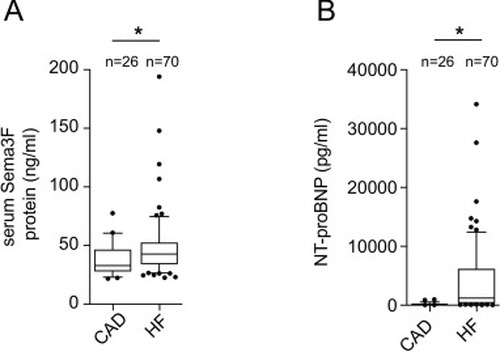
Sema3F serum levels are increased in patients with heart failure. (A) Serum Sema3F was measured by means of ELISA in CAD patients (n = 26) and HF patients (n = 70). Sema3F levels were higher in HF patients in comparison to CAD. The results were analyzed using the Mann-Whitney test. A p-value of <0.05 is considered significant and marked with an asterisk (*). (B) NT-proBNP levels of HF patients (n = 70) were significantly higher than those of CAD (n = 26) patients. The results were analyzed using the Mann-Whitney test. A p-value <0.05 is considered to be significant (*p < 0.05). |
|
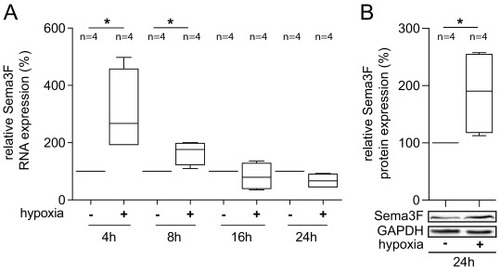
Sema3F expression is regulated by hypoxia in cardiac endothelial cells. (A) The expression of Sema3F RNA was increased in endothelial cells (HCECs) under hypoxic conditions, with the highest levels being observed after 4 h. HCECs were exposed to hypoxia (1 % O2) for the indicated times, after which the cells were harvested, RNA was isolated and reverse transcribed. cDNA was then used for the quantification of Sema3F expression by qRT-PCR. The data presented here is derived from four independent experiments (biological replicates, n = 4), and the statistical analysis employed was the Man-Whitney test. A p-value <0.05 is considered to be significant and marked with an asterisk (*). (B) Sema3F protein was significantly increased in response to 24 h of hypoxia in comparison to normoxia. For Western blot analysis HCECs were lysed and Sema3F protein levels were detected by an anti-Sema3F antibody. GAPDH was used as a loading control. Representative blots from four independent experiments are presented (biological replicates, n = 4). The results were analyzed using the Mann-Whitney test (*p < 0.05). |
|
Serum from HF patients inhibits sprouting and branching in endothelial cells, and Sema3F levels in serum from HF patients correlate with its anti-angiogenic effect HUVECs were incubated for 16 to 18 h with 30 % serum from patients with CAD (n = 10), HF (n = 21) or EBM with 2 % FCS (control, n = 12) and then assayed on Matrigel® for 2.5 h. (A) Cumulative sprout length and (B) branch points of capillary-like structures were diminished in response to HF serum compared to treatment with serum from CAD subjects. Box plots extend from the 25th to the 75th percentile with the median marked. Whiskers extend from the 10th to the 90th percentile. The results were analyzed using the one-way ANOVA test followed by Bonferroni's multiple comparisons test: *p < 0.05 CAD vs. controls or CAD vs. HF (n, number of patients). (C) Serum Sema3F levels were significantly higher in patients with HF (n = 21) compared to patients with CAD (n = 10). Box plots extend from the 25th to the 75th percentile with the median marked. Whiskers extend from the 10th to the 90th percentile. Mann-Whitney test, *p < 0.05 CAD vs HF. (D) The cumulative sprouting length failed to attain statistical significance (Spearman's r = 0.4180, p = 0.0666), the branch points of the Matrigel® wells that had been treated with serum from HF patients (n = 21) exhibited an inverse correlation with serum Sema3F concentration (E, Spearman's r - 0.4543, p < 0.05). |
|
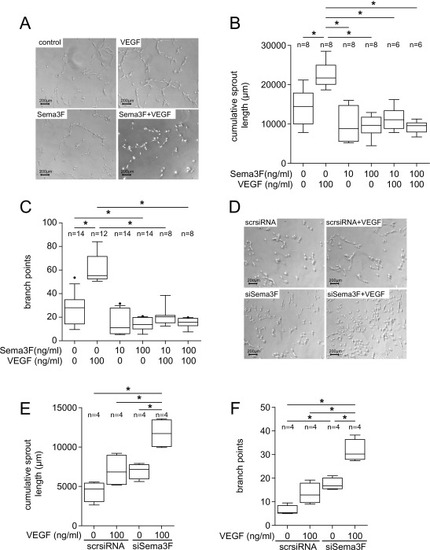
Sema3F is an inhibitor of angiogenesis in Matrigel® sprouting assays. (A-C) In cardiac endothelial cells (HCECs) Sema3F abolished the pro-angiogenic effect of VEGF. HCECs were cultured with the indicated amounts of Sema3F or VEGF alone or in combination over 16 to 18 h. HCECs were then assayed on Matrigel® for further 2.5 h. (A) Representative micrographs of stimulated HCECs are shown. (B&C) Sema3F prevented VEGF-induced cumulative sprout length (B) and branching of HCECs (C). Data from 4 independent experiments were shown (n, number of biological replicates). The results were analyzed using the one-way ANOVA and Bonferroni's multiple comparisons test: *p < 0.05. Figs. Dsingle bondF show the effect of Sema3F knockdown on angiogenesis of HCECs. The siRNA-based Sema3F knockdown resulted in a potentiated pro-angiogenic effect of VEGF. HCECs transfected with Sema3F-specific siRNA (siSema3F) or control siRNA (ctrl-siRNA) were treated with 100 ng/ml VEGF or EBM with 2 % FCS for 18 h before being assayed on Matrigel®. (D) Representative micrographs are shown. Sema3F knockdown led to a significant increase in cumulative sprout length (E) and number of branch points (F). (E&F) Data from 4 independent experiments were shown (n, number of biological replicates). All box plots extend from the 25th to the 75th percentile with the median marked. Whiskers extend from the 10th to the 90th percentile. The results were analyzed using one-way ANOVA followed by Bonferroni's multiple comparison test: *p < 0.05. |
|
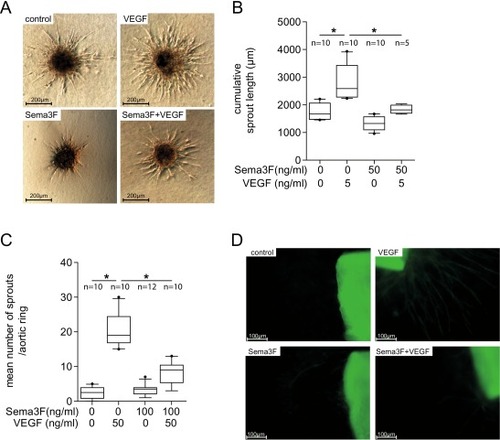
Sema3F inhibits angiogenesis in spheroid sprouting and aortic ring assay. (A&B) For the Spheroid sprouting assay, HCECs were formed to spheroids and were then stimulated for 24 h with the indicated amounts of Sema3F, VEGF or both. (A) Representative micrographs are shown. (B) Sema3F caused a significant decrease in sprout length and reduced the proangiogenic effect of VEGF. Data from 4 independent experiments are shown. Ten spheroids per well per condition were measured and the average cumulative sprout length was estimated (n, number of biological replicates). Analysis with one-way ANOVA followed by Bonferroni's multiple comparisons test. *p < 0.05. (C&D) In the aortic ring assay, aortic rings were stimulated with the indicated amounts of Sema3F, VEGF or both on days 3, 5 and 7. The number of capillaries was counted on day 9. (C) Compared to controls, VEGF alone increased sprouting, while co-treatment of aortic rings with Sema3F prevented VEGF-induced sprouting ex vivo. Data from 3 independent experiments, total of 10–12 aortic rings per group (n, number of biological replicates). All box plots extend from the 25th to the 75th percentile with the median marked. Whiskers extend from the 10th to the 90th percentile. The results were analyzed using one-way ANOVA followed by Bonferroni's multiple comparisons test. *p < 0.05. |
|
Sema3F is essential for the proper angiogenesis in zebrafish. Zebrafish embryos in 1- to 2-cell stage were injected with Sema3F-specific morpholino (Sema3Fa-(MO1-S3fa), Sema3Fb-morpholino (MO1-S3fb), or both). Uninjected and control-morpholino-injected embryos served as controls. (A-O) Representative pictures of zebrafish embryos in the transmitted light and fluorescent light microscopy were taken on 2nd day post fertilization (dpf). (P) On the 1st dpf, Sema3F morphants showed a higher mortality rate than zebrafish injected with control morpholino. On the 2nd dpf the larvae were dechorionated and examined for phenotypes. Sema3F morphants showed typical vessel ectasia in the distal trunk (Q), intersegmental vessel (ISV) malformations (R), as well as increased occurrence of pericardial effusions (S). All these features were observed most frequently in Sema3Fb morphants. (Q-R) The data presented are from 4 independent experiments, the total number (n) of zebrafish is shown in the figures. All box plots extend from the 25th to the 75th percentile, with the median marked. Whiskers extend from the 10th to the 90th percentile. Results were analyzed by one-way ANOVA followed by Bonferroni's multiple comparison test: *p < 0.05. |
|
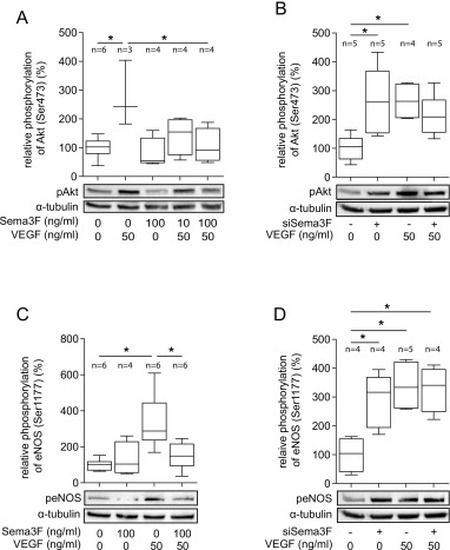
Sema3F inhibits VEGF-induced phosphorylation of Akt and eNOS. (A) Sema3F inhibits VEGF-mediated phosphorylation of Akt at Ser473. HUVECs were treated with VEGF (50 ng/ml) and Sema3F (10 ng/ml or 100 ng/ml) or solvent for 30 min. HUVECs were lysed and proteins were extracted to perform Western blot analysis of Akt, pAkt (Ser473) and α-tubulin. α-tubulin served as the loading control. Bands were quantified by densitometric analysis. Representative blots from four independent experiments are shown (n, number of biological replicates). (B) SiRNA-mediated Sema3F knockdown increased phosphorylation of Akt at baseline. HUVECs were transfected with Sema3F siRNA or scrambled siRNA (scrsiRNA). 24 h post transfection cells were treated with VEGF (50 ng/ml) or solvent for 15 min. Cell lysates were used for Western blotting. Representative blots from five independent experiments are shown (n, number of biological replicates). (C) Sema3F inhibits VEGF-induced phosphorylation of eNOS at Ser1177. HUVECs were incubated with VEGF (50 ng/ml), Sema3F (100 ng/ml) or both for 15 min and then lysed. Phosphorylation of eNOS (ser1177) was analyzed by Western blotting with an anti-phospho-eNOS antibody. α-tubulin served as loading control. Representative blots from four independent experiments are shown (n, number of biological replicates). (D) SiRNA-mediated Sema3F knockdown enhanced phosphorylation of eNOS at baseline. HUVECs were transfected with siSema3F or scrsiRNA. 24 h later the cells were stimulated with or without VEGF (50 ng/ml) for 15 min. Representative data from four independent experiments are shown (n, number of biological replicates). (A-D) Data were analyzed by the one-way ANOVA test followed by Bonferroni's multiple comparisons test. *p < 0.05. All box plots extend from the 25th to the 75th percentile with the median marked. Whiskers extend from the 10th to the 90th percentile. |