- Title
-
Paving the way for better ototoxicity assessments in cisplatin therapy using more reliable animal models
- Authors
- Manickam, V., Zallocchi, M.
- Source
- Full text @ Front. Cell. Neurosci.
|
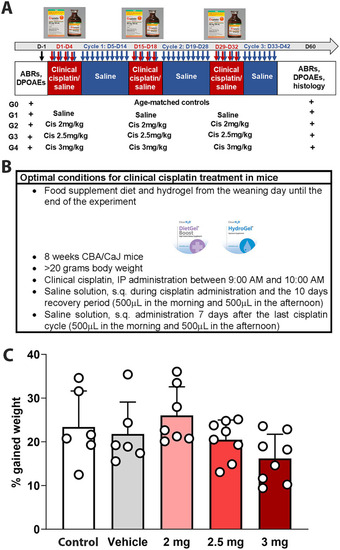
Experimental design for multi-cycle clinical cisplatin protocol. |
|
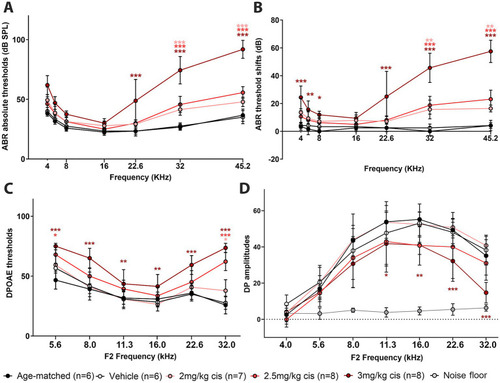
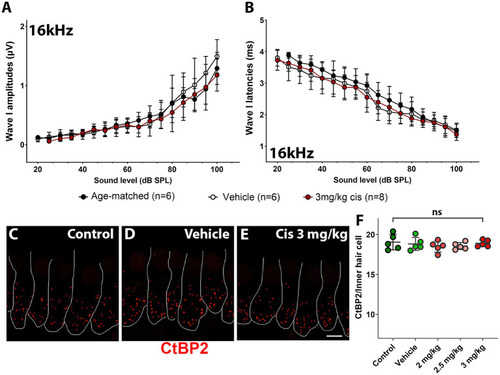
Clinical cisplatin affects hearing function in mice in a dose-dependent manner. Eight weeks old mice (>20 grams) received clinical cisplatin in a multi-cycle protocol. Hearing function was measured before and after the completion of cisplatin treatment. The following groups were tested: age-matched controls (black circles), vehicle (saline solution, white circles), clinical cisplatin at 2 mg/kg b.w. (pink circles), 2.5 mg/kg b.w (red circles) and 3 mg/kg b.w. (marron circles). |
|
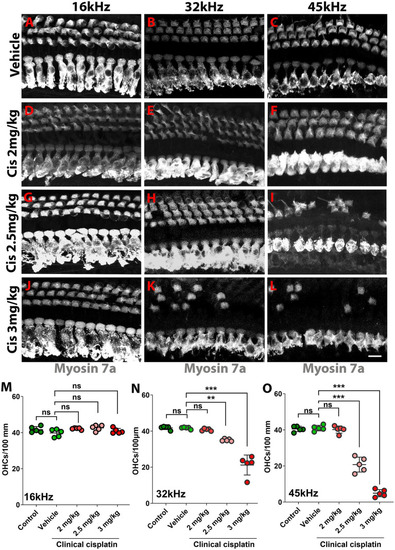
Clinical cisplatin treatment results in hair cell damage. |
|
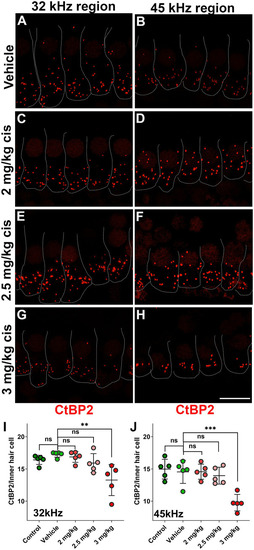
Clinical cisplatin treatment results in pre-synaptic ribbon loss at higher frequencies. |
|
Assessment of clinical cisplatin treatment at the 16 kHz frequency region. Wave I amplitudes |
|
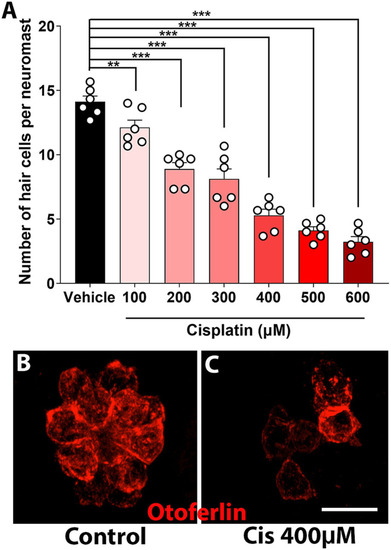
Dose–response curve of clinical cisplatin in zebrafish. |
|
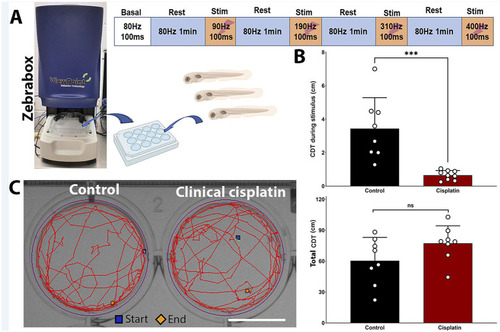
Clinical cisplatin treatment affects stimulus-dependent swimming behavior. |