|
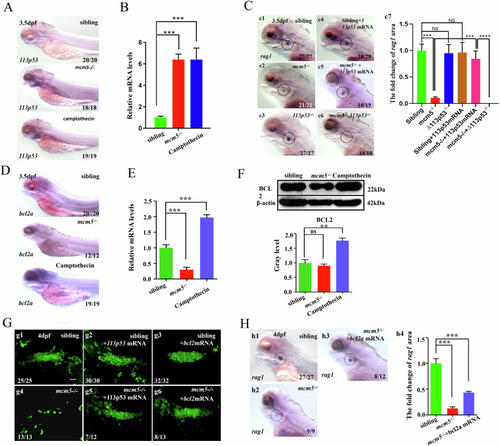
Silencing of bcl2a in mcm5 mutants accelerates the death of immature T lymphocytes. A Upregulation of Δ113p53 expression in mcm5-/ -embryos and embryos treated with camptothecin was confirmed using in situ experiments. B RT‒qPCR examination showed that the expression of Δ113p53 was upregulated in thymus region of mcm5−/− embryos and embryos treated with camptothecin. C The expression of rag1 in controls (c1, n = 27), Δ113p53−/− embryos (c3, n = 27) and embryos injected with Δ113p53 mRNA (c4, n = 28) was normal (c7). Downregulation of rag1 expression in mcm5−/−embryos (c2, n = 21) was partially reversed by injection of Δ113p53 mRNA (c5, n = 19; c7) and was exacerbated in mcm5−/−;Δ113p53−/−double mutants (c6, n = 18; c7). D, E The expression of bcl2a in different embryos was assessed using WISH (D) and RT‒qPCR (E). F At the protein level, Bcl2 was upregulated in embryos treated with camptothecin but not in mcm5−/− embryos. G In the transgenic line Tg(coro1a:GFP), compared to that in siblings (g1, n = 25), mcm5−/− embryos (g4, n = 13) and embryos injected with Δ113p53 mRNA (g2, n = 30), the decrease in coro1a:GFP cells in mcm5−/− embryos was partially rescued by injection of Δ113p53 mRNA (g5, n = 12). Overexpression of bcl2a mRNA did not affected the number of Coro1a:GFP labeled cells (g3, n = 32), but also partially reversed the T-cell developmental defect in mcm5−/−embryos (g6, n = 13). H Compared to that in siblings (h1, n = 27), mcm5−/− embryos (h2, n = 9), the expression of rag1 was partially rescued by injection of bcl2 mRNA in mcm5−/− embryos (h3, n = 12; h4). Scale bars, 40 μm. For (B, c7, E and f4), the data were presented as means ± SD; The P-values (t-test; two-tailed); NS not significant. “**” P < 0.01, “***”P < 0.001.
|