- Title
-
Prime editing outperforms homology-directed repair as a tool for CRISPR-mediated variant knock-in in zebrafish
- Authors
- Vanhooydonck, M., De Neef, E., De Saffel, H., Boel, A., Willaert, A., Callewaert, B., Claes, K.B.M.
- Source
- Full text @ Lab Anim (NY)
|
Comparison of EE and IF obtained for each target by microinjection of Alt-R HDR components with 100, 200, 400 or 800 pg Cas9 into the yolk of one-cell-stage zebrafish embryos. Left-hand y axes show EE (blue) and right-hand y axes show IF (gray). The bars and error bars represent the mean + s.e.m. of three biologically independent replicates (n = 3), where each replicate represents a pool of 20–30 injected embryos. Tukey’s multiple-comparisons test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. |
|
Comparison of EE and IF obtained for each target by microinjection of Alt-R HDR components into the cell or the yolk.Zebrafish embryos at the one-cell stage were injected with 200 pg (col1a2 c.918A>T), 400 pg (col1a2 c.1416A>C, col1a2 c.2093G>A) or 800 pg (atp6v1e1b c.634_636CGA>TGG, atp6v1e1b c.383T>C, brca2 c.6803G>A) Cas9 into the cell or the yolk. Left-hand y axes show EE (blue) and right-hand y axes show IF (gray). Bars and error bars represent the mean + s.e.m. of three biologically independent replicates (n = 3), where each replicate represents a pool of 20–30 injected embryos. Unpaired t-test. |
|
Comparison of EE and IF obtained for each target by microinjection of Alt-R HDR templates, Alt-R HDR templates with silent guide-blocking variants or unmodified HDR templates.Zebrafish embryos at the one-cell stage were injected with 200 pg (col1a2 c.918A>T), 400 pg (col1a2 c.1416A>C, col1a2 c.2093G>A) or 800 pg (atp6v1e1b c.634_636CGA>TGG, atp6v1e1b c.383T>C, brca2 c.6803G>A) Cas9 into the yolk. Left-hand y axes show EE (blue) and right-hand y axes show IF (gray). The bars and error bars represent the mean + s.e.m. of three biologically independent replicates (n = 3), where each replicate represents a pool of 20–30 injected embryos. Dunnett’s multiple-comparisons test: *P < 0.05, **P < 0.01. |
|
Comparison of EE and IF obtained for each target by microinjection of Alt-R HDR components and PE components. Zebrafish embryos at the one-cell stage were injected with 200 pg (col1a2 c.918A>T), 400 pg (col1a2 c.1416A>C, col1a2 c.2093G>A) or 800 pg (atp6v1e1b c.634_636CGA>TGG, atp6v1e1b c.383T>C, brca2 c.6803G>A) Cas9. EE and IF were compared with injection of PE components (RNP mixture containing 480 ng/µL pegRNA and 1,380 ng/µL PE2-His protein) into the yolk of one-cell-stage zebrafish embryos. Left-hand y axes show EE (blue) and right-hand y axes show IF (gray). The bars and error bars represent the mean + s.e.m. of three biologically independent replicates (n = 3), where each replicate represents a pool of 20–30 injected embryos. Unpaired two-tailed t-test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. |
|
F0 founder screen of 20 potential adult founder zebrafish generated by microinjection of HDR or PE components into the yolk of one-cell-stage zebrafish embryos. a, Results for zebrafish injected with Alt-R HDR components with 200 pg (col1a2 c.918A>T), 400 pg (col1a2 c.1416A>C, col1a2 c.2093G>A) or 800 pg (atp6v1e1b c.634_636CGA>TGG, atp6v1e1b c.383T>C, brca2 c.6803G>A) Cas9. b, Results for zebrafish injected with PE components (RNP mixture containing 480 ng/µL pegRNA and 1,380 ng/µL PE2-His protein). |
|
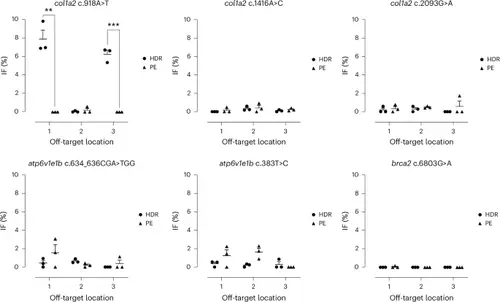
IF at top three predicted off-target locations. IF was determined for Alt-R HDR components with 200 pg (col1a2 c.918A>T), 400 pg (col1a2 c.1416A>C, col1a2 c.2093G>A) or 800 pg (atp6v1e1b c.634_636CGA>TGG, atp6v1e1b c.383T>C, brca2 c.6803G>A) Cas9 or PE components (RNP mixture containing 480 ng/µL pegRNA and 1,380 ng/µL PE2-His protein) injected into the yolk of one-cell-stage zebrafish embryos. The bars and error bars represent the mean + s.e.m. of three biologically independent replicates (n = 3), where each replicate represents a pool of 20–30 injected embryos. Unpaired two-tailed t-test: **P < 0.01, ***P < 0.001. |