- Title
-
The P4-phospholipid flippase Atp11a is required for maintenance of eye and ear structure in zebrafish
- Authors
- Hawkey-Noble, A., Tobin, C., Ameen, M., Osmond, L., Gill, C., Bottaro, C.S., Young, T.L., French, C.R.
- Source
- Full text @ J. Cell Sci.
|
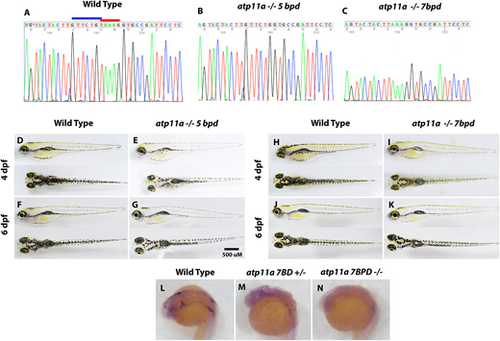
Generation of atp11a loss-of-function mutants. (A–C) Two mutant strains were isolated using the same gRNA, creating a 7 bp deletion (GTTCTGT) highlighted by the blue bar and a 5 bp deletion (TAAAG) highlighted by the red bar. (D–K) At both 4 and 6 dpf, larvae are morphologically wild type, inflate their swim bladders and have reduced pigment. (L–N) In situ hybridization using an antisense atp11a probe demonstrates reduced transcript levels in heterozygous and homozygous mutant embryos at 24 hpf. |
|
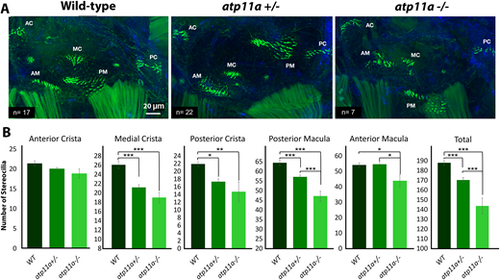
Reduced number of stereocilia in atp11a mutants. (A) Phalloidin staining of F-actin filaments in the hair cells of the atp11anl1005 zebrafish inner ear at 5 dpf, illustrating significant hair cell loss in the medial crista (MC), posterior crista (PC) and macula (PM), as well as the anterior macula (AM), resulting in an overall decrease in the inner ear. (B) Hair cell quantification for the anterior crista (AC), anterior macular, medial crista, posterior crista and posterior macula demonstrates a reduction in stereocilia in heterozygous and homozygous mutants. Data are mean±s.e.m. with significance testing (*P<0.05, **P<0.01, ***P<0.001) calculated using a one-factor ANOVA with Tukey's post-hoc analysis for multiple comparisons. Wild type, n=17; heterozygous, n=22; homozygous mutant, n=7. |
|
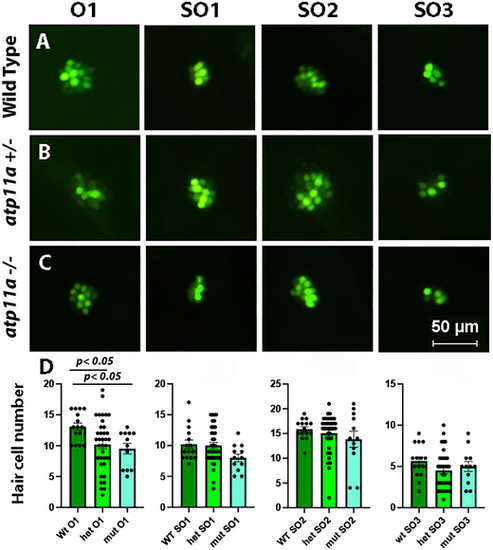
Reduced number of hair cells in the O1 neuromast. (A–C) Nuclear staining of four neuromasts in 5 dpf atp11anl1007 larvae with Yo-Pro-1, demonstrating a decrease in the number of positively stained cells in the O1 neuromast in homozygous and heterozygous mutants compared with wild type. (D) Neuromast hair cell quantification for one otic (O1) and three supraoptic (SO1, SO2 and SO3) neuromasts demonstrates a significant reduction in hair cell number for the O1 neuromast in homozygous and heterozygous mutants. Data are mean±s.e.m. with significance calculated using a one-factor ANOVA with Tukey's post-hoc analysis for multiple comparisons. Wild type, n=15; heterozygous, n=36; homozygous mutant, n=12. PHENOTYPE:
|
|
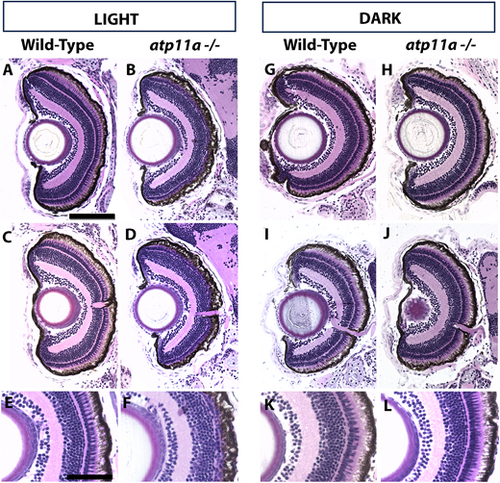
Photoreceptor loss in atp11anl1007 mutants is partially light dependent. (A,C,E) Wild-type larvae display regular retinal lamination with a distinct photoreceptor layer. (B,D,F) atp11anl1007 larvae raised in ambient light display a reduced photoreceptor layer. (G–L) When raised in the dark, both wild-type (G,I,K) and atp11anl1007 larvae (H,J,L) have a more wild-type appearing photoreceptor layer. Wild type light, n=9; homozygous mutant light, n=7; wild type dark, n=8; homozygous mutant dark, n=9. All larvae are at 6 dpf. Scale bars: 100µm in A for A–D,G-J; 50 µm in E for E,F,K,L. PHENOTYPE:
|
|
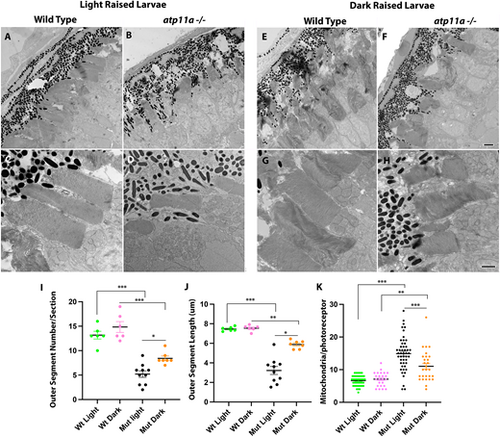
Ultrastructure analysis of atp11anl1007 mutant photoreceptors. (A–D) Photoreceptors in ultrathin sections show a reduction of outer segments in homozygous mutants when compared to wild-type siblings. (E–H) This phenotype partially resolves when mutant larvae are raised in the dark. Quantification of outer segment number (I), length (J) and mitochondrial number (K) confirm a partial rescue via dark-rearing conditions. Data are mean±s.e.m. ***P<0001, **P<0.001, *P<0.01 (one-factor ANOVA with Tukey’s analysis for multiple comparisons). Wild type light, n=6; mutant light, n=6; wild type dark, n=3; mutant dark, n=3. Larvae are at 6 dpf. Scale bars: 2 µm in F for A,B,E,F; 1 µm in H for C,D,G,H. PHENOTYPE:
|
|
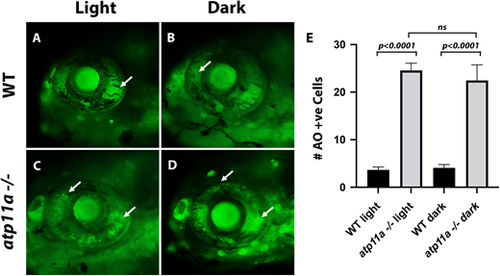
Cell death in atp11anl1007 mutant eyes. (A,B) Few dying cells (Acridine Orange positive, arrows) are observed in wild-type sibling eyes in light and dark conditions. (C,D) In atplla homozygous mutants, an increase in the number of Acridine Orange-positive cells (arrows) is observed in light and dark conditions. (E) Quantification of cell death (number of Acridine Orange-positive cells), presented as mean±s.e.m. Wild type light, n=9; homozygous mutant light, n=9; wild type dark, n=10; homozygous mutant dark, n=11. Significance testing was carried out using two-factor ANOVA. All larvae are at 6 dpf. PHENOTYPE:
|
|
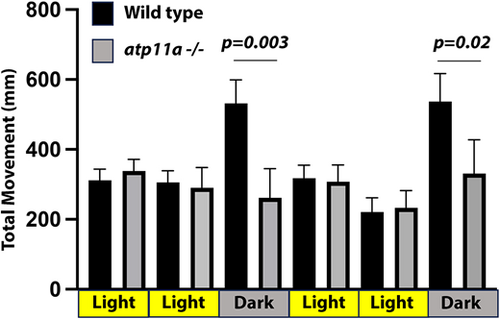
atp11a mutants have altered swimming behavior. When transitioning from light to dark, wild-type larvae have a stereotypical increase in swimming activity, which is not observed in atp11anl1007 mutant larvae. Wild type, n=11; mutant, n=7. Data are mean±s.d. Significance testing was carried out using a one-factor ANOVA with Tukey's analysis of multiple comparisons. Analysis was performed at 7 dpf. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

Unillustrated author statements PHENOTYPE:
|