Characterization of na(+) and ca(2+) channels in zebrafish dorsal root ganglion neurons
- Authors
- Won, Y.J., Ono, F., and Ikeda, S.R.
- ID
- ZDB-PUB-120813-12
- Date
- 2012
- Source
- PLoS One 7(8): e42602 (Journal)
- Registered Authors
- Ono, Fumihito
- Keywords
- none
- MeSH Terms
-
- Receptors, GABA-B/metabolism
- Neurons/cytology
- Neurons/metabolism*
- Zebrafish Proteins/genetics
- Zebrafish Proteins/metabolism
- PubMed
- 22880050 Full text @ PLoS One
Background
Dorsal root ganglia (DRG) somata from rodents have provided an excellent model system to study ion channel properties and modulation using electrophysiological investigation. As in other vertebrates, zebrafish (Danio rerio) DRG are organized segmentally and possess peripheral axons that bifurcate into each body segment. However, the electrical properties of zebrafish DRG sensory neurons, as compared with their mammalian counterparts, are relatively unexplored because a preparation suitable for electrophysiological studies has not been available.
Methodology/Principal Findings
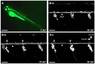
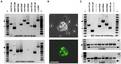
We show enzymatically dissociated DRG neurons from juvenile zebrafish expressing Isl2b-promoter driven EGFP were easily identified with fluorescence microscopy and amenable to conventional whole-cell patch-clamp studies. Two kinetically distinct TTX-sensitive Na+ currents (rapidly- and slowly-inactivating) were discovered. Rapidly-inactivating INa were preferentially expressed in relatively large neurons, while slowly-inactivating INa was more prevalent in smaller DRG neurons. RT-PCR analysis suggests zscn1aa/ab, zscn8aa/ab, zscn4ab and zscn5Laa are possible candidates for these INa components. Voltage-gated Ca2+ currents (ICa) were primarily (87%) comprised of a high-voltage activated component arising from ω-conotoxin GVIA-sensitive CaV2.2 (N-type) Ca2+ channels. A few DRG neurons (8%) displayed a miniscule low-voltage-activated component. ICa in zebrafish DRG neurons were modulated by neurotransmitters via either voltage-dependent or -independent G-protein signaling pathway with large cell-to-cell response variability.
Conclusions/Significance
Our present results indicate that, as in higher vertebrates, zebrafish DRG neurons are heterogeneous being composed of functionally distinct subpopulations that may correlate with different sensory modalities. These findings provide the first comparison of zebrafish and rodent DRG neuron electrical properties and thus provide a basis for future studies.